
伤口世界

- 星期六, 07 3月 2026
Blood oxygen saturation is lower in persons with pre-diabetes and screen-detected diabetes compared with non-diabetic individuals: A population-based study of the Lolland-Falster Health Study cohort
Jens Christian Laursen1*, Randi Jepsen2 , Neda Esmailzadeh Bruun-Rasmussen2 , Marie Frimodt-Møller1 , Marit Eika Jørgensen3 , Peter Rossing1,4 and Christian Stevns Hansen1
1 Complications Research, Steno Diabetes Center Copenhagen, Herlev, Denmark,
2Center for Epidemiological Research, Nykøbing Falster Hospital, Nykøbing Falster, Denmark,
3Steno Diabetes Center Greenland, Nuuk, Greenland, 4Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Aims: Low blood oxygen saturation is associated with increased mortality and persons with diabetes have sub-clinical hypoxemia. We aimed to confirm the presence of sub-clinical hypoxemia in pre-diabetes, screen-detected diabetes and known diabetes.
Methods: Pre-diabetes was defined as hemoglobin A1C (HbA1C) ≥ 42 mmol/mol and <48 mmol/mol; known diabetes as history or treatment of diabetes; screen-detected diabetes as no history or treatment of diabetes and HbA1C ≥ 48 mmol/mol. Blood oxygen saturation was measured with pulse oximetry. Urine albumin-to creatinine ratio (UACR) was measured on a single spot urine.
Results: The study included 829 adults (≥18 years) with diabetes (713 (86%) with known diabetes; 116 (14%) with screen-detected diabetes) and 12,747 without diabetes (11,981 (94%) healthy controls; 766 (6%) with pre-diabetes). Mean (95% CI) blood oxygen saturation was 96.3% (96.3% to 96.4%) in diabetes which was lower than in non-diabetes [97.3% (97.2–97.3%)] after adjustment for age, gender, and smoking (p < 0.001), but significance was lost after adjustment for BMI (p = 0.25). Sub-groups with pre-diabetes and screen-detected diabetes had lower blood oxygen saturations than healthy controls (p-values < 0.01). Lower blood oxygen saturation was associated with higher UACR.
Conclusions: Persons with pre-diabetes and screen-detected diabetes have sub-clinical hypoxemia, which is associated with albuminuria.
KEYWORDS
hypoxia, microvascular complications, albuminuria, type 2 diabetes, pre-diabetes

- 星期四, 05 3月 2026
Non-invasive type 2 diabetes risk scores do not identify diabetes when the cause is β-cell failure: The Africans in America study
Annemarie Wentzel1,2,3*, Arielle C. Patterson1 , M. Grace Duhuze Karera1,4,5, Zoe C. Waldman1 , Blayne R. Schenk1 , Christopher W. DuBose1 , Anne E. Sumner1,4 and Margrethe F. Horlyck-Romanovsky1,6*
1 Section on Ethnicity and Health, Diabetes, Endocrinology, and Obesity Branch, National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, United States,
2 Hypertension in Africa Research Team, North-West University, Potchefstroom, South Africa, 3South African Medical Research Council, Unit for Hypertension and Cardiovascular Disease, North-West University, Potchefstroom, South Africa, 4National Institute of Minority Health and Health Disparities, National Institutes of Health, Bethesda, MD, United States, 5 Institute of Global Health Equity Research, University of Global Health Equity, Kigali, Rwanda, 6Department of Health and Nutrition Sciences, Brooklyn College, City University of New York, New York, NY, United States
Background: Emerging data suggests that in sub-Saharan Africa β-cell-failure in the absence of obesity is a frequent cause of type 2 diabetes (diabetes). Traditional diabetes risk scores assume that obesity-linked insulin resistance is the primary cause of diabetes. Hence, it is unknown whether diabetes risk scores detect undiagnosed diabetes when the cause is β-cell-failure.
Aims: In 528 African-born Blacks living in the United States [age 38 ± 10 (Mean ± SE); 64% male; BMI 28 ± 5 kg/m2 ] we determined the: (1) prevalence of previously undiagnosed diabetes, (2) prevalence of diabetes due to β-cell-failure vs. insulin resistance; and (3) the ability of six diabetes risk scores [Cambridge, Finnish Diabetes Risk Score (FINDRISC), Kuwaiti, Omani, Rotterdam, and SUNSET] to detect previously undiagnosed diabetes due to either β-cell-failure or insulin resistance.
Methods: Diabetes was diagnosed by glucose criteria of the OGTT and/or HbA1c ≥ 6.5%. Insulin resistance was defined by the lowest quartile of the Matsuda index (≤2.04). Diabetes due to β-cell-failure required diagnosis of diabetes in the absence of insulin resistance. Demographics, body mass index (BMI), waist circumference, visceral adipose tissue (VAT), family medical history, smoking status, blood pressure, antihypertensive medication, and blood lipid profiles were obtained. Area under the Receiver Operator Characteristics Curve (AROC) estimated sensitivity and specificity of each continuous score. AROC criteria were: Outstanding: >0.90; Excellent: 0.80–0.89; Acceptable: 0.70–0.79; Poor: 0.50–0.69; and No Discrimination: 0.50.
Results: Prevalence of diabetes was 9% (46/528). Of the diabetes cases, β-cell-failure occurred in 43% (20/46) and insulin resistance in 57% (26/46). The β-cell-failure group had lower BMI (27 ± 4 vs. 31 ± 5 kg/m2 P < 0.001), lower waist circumference (91 ± 10 vs. 101 ± 10cm P < 0.001) and lower VAT (119 ± 65 vs. 183 ± 63 cm3 , P < 0.001). Scores had indiscriminate or poor detection of diabetes due to β-cell-failure (FINDRISC AROC = 0.49 to Cambridge AROC = 0.62). Scores showed poor to excellent detection of diabetes due to insulin resistance, (Cambridge AROC = 0.69, to Kuwaiti AROC = 0.81).
Conclusions: At a prevalence of 43%, β-cell-failure accounted for nearly half of the cases of diabetes. All six diabetes risk scores failed to detect previously undiagnosed diabetes due to β-cell-failure while effectively identifyingdiabetes when the etiology was insulin resistance. Diabetes risk scores whichcorrectly classify diabetes due to B-cell-failure are urgently needed.
KEYWORDS
type 2 diabetes, risk score, African (Black) diaspora, β-cell failure, insulin resistance, diabetes screening

- 星期一, 02 3月 2026
Implantable biomedical materials for treatment of bone infection
Wang Shuaishuai 1† , Zhu Tongtong1† , Wang Dapeng2 ,
Zhang Mingran1 , Wang Xukai 1 , Yu Yue1 , Dong Hengliang1 ,
Wu Guangzhi 1 * and Zhang Minglei 1 *
1 Department of Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, China,
2 Department of Orthopedics, Siping Central Hospital, Siping, China
EDITED BY
Xiaoyuan Li,
Northeast Normal University, China
REVIEWED BY
Fuzeng Ren,
Southern University of Science and
Technology, China
Gong Cheng,
Harvard University, United States
Ruogu Qi,
Nanjing University of Chinese Medicine,
China
*CORRESPONDENCE
Wu Guangzhi,
该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。
Zhang Minglei,
该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。
†
These authors have contributed equally to this work
SPECIALTY SECTION
This article was submitted to Biomaterials, a section of the journal
Frontiers in Bioengineering and Biotechnology
RECEIVED 27 October 2022
ACCEPTED 18 January 2023
PUBLISHED 30 January 2023
CITATION
Shuaishuai W, Tongtong Z, Dapeng W, Mingran Z, Xukai W, Yue Y, Hengliang D, Guangzhi W and Minglei Z (2023), Implantable biomedical materials for treatment of bone infection.
Front. Bioeng. Biotechnol. 11:1081446.
doi: 10.3389/fbioe.2023.1081446
COPYRIGHT
© 2023 Shuaishuai, Tongtong, Dapeng, Mingran, Xukai, Yue, Hengliang, Guangzhi and Minglei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
The treatment of bone infections has always been difficult. The emergence of drugresistant bacteria has led to a steady decline in the effectiveness of antibiotics. It is also especially important to fight bacterial infections while repairing bone deffects and cleaning up dead bacteria to prevent biofilm formation. The development of biomedical materials has provided us with a research direction to address this issue.
We aimed to review the current literature, and have summarized multifunctional antimicrobial materials that have long-lasting antimicrobial capabilities that promote angiogenesis, bone production, or “killing and releasing.” This review provides a comprehensive summary of the use of biomedical materials in the treatment of bone infections and a reference thereof, as well as encouragement to perform further research in this field.
KEYWORDS
biological materials, bone infection, multifunctional material, implantable material, treatment of bone infection, progress of infection treatment, multifunctionalization of materials

- 星期六, 28 2月 2026
Diabetes duration and types of diabetes treatment in data-driven clusters of patients with diabetes


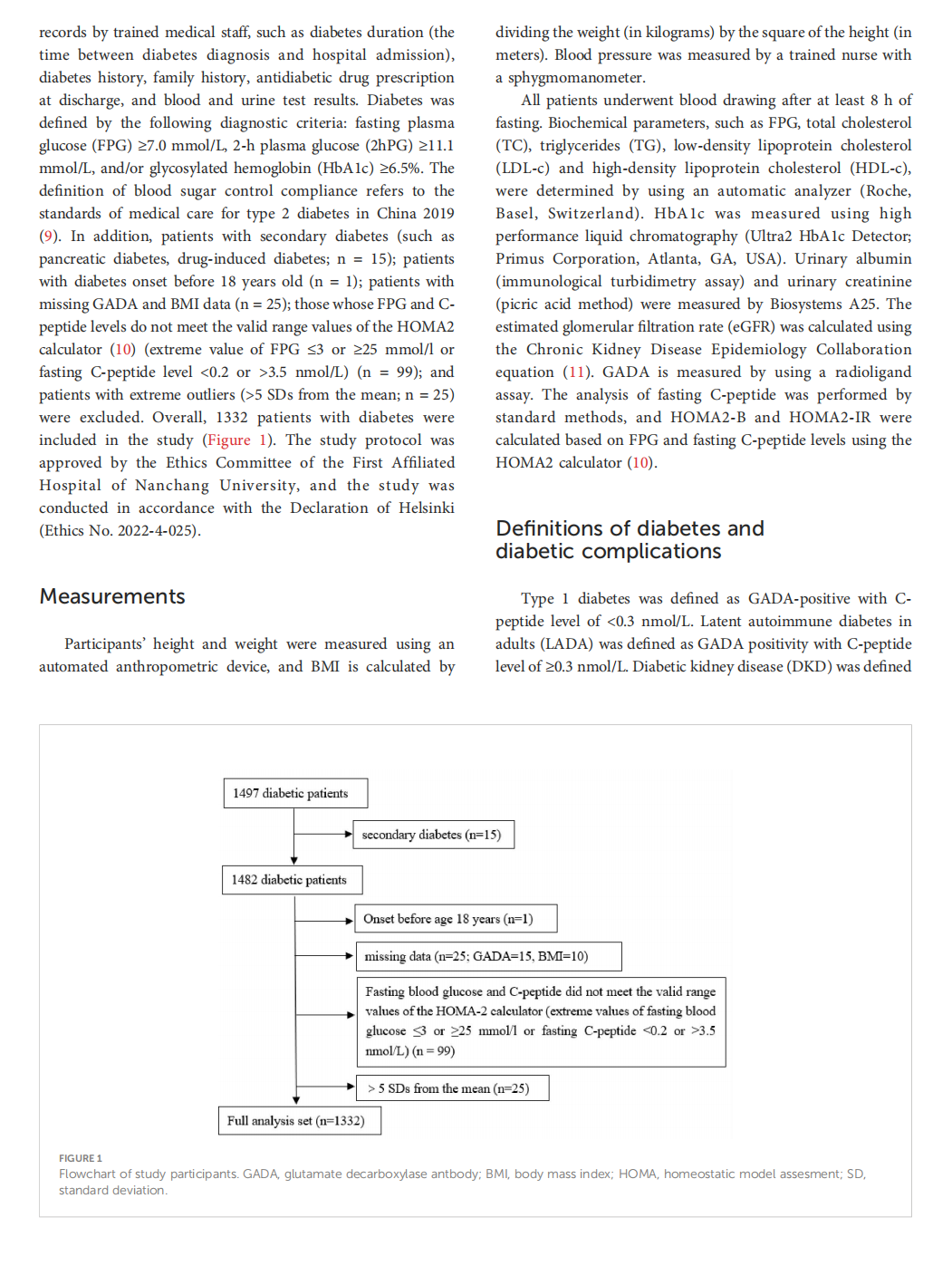

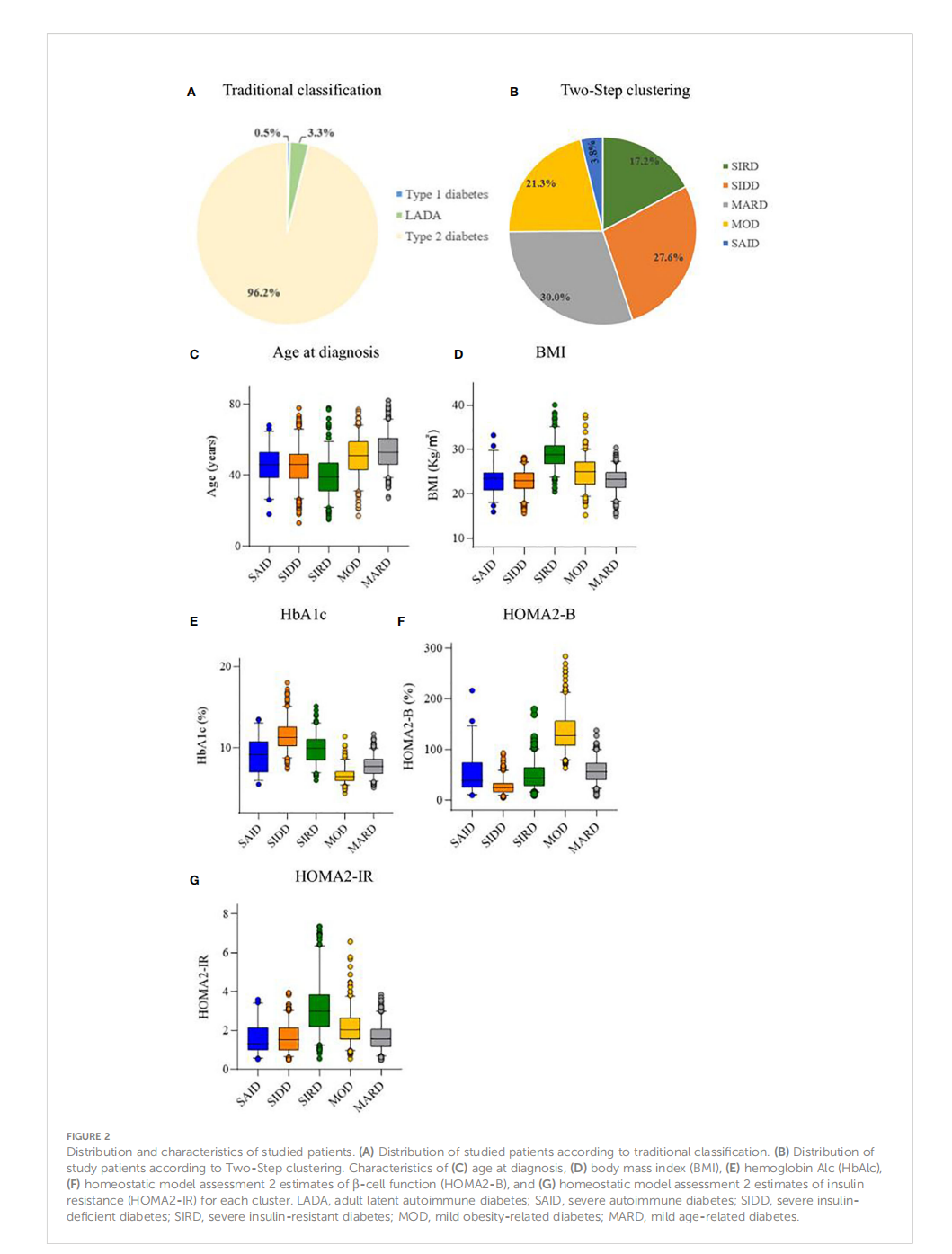
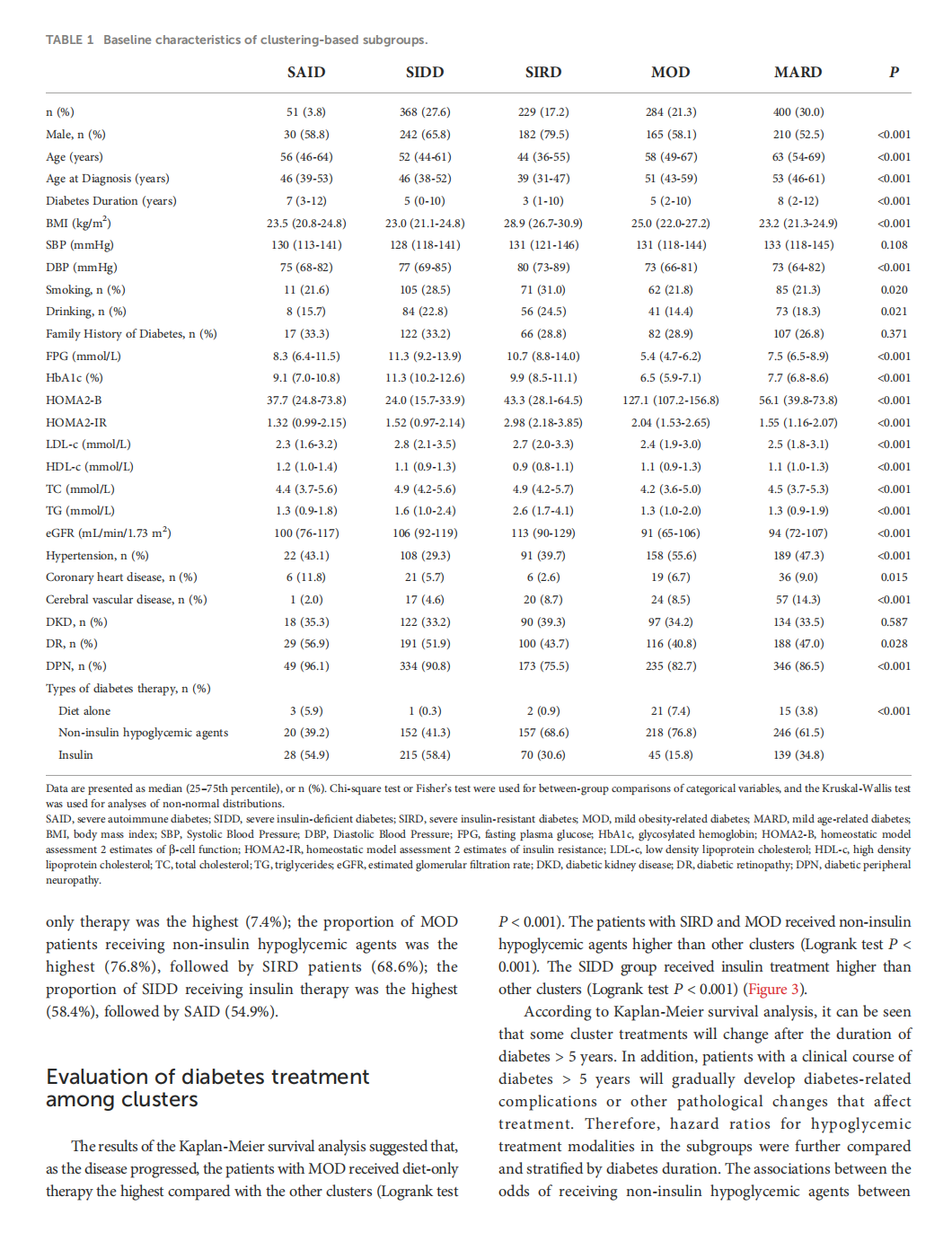
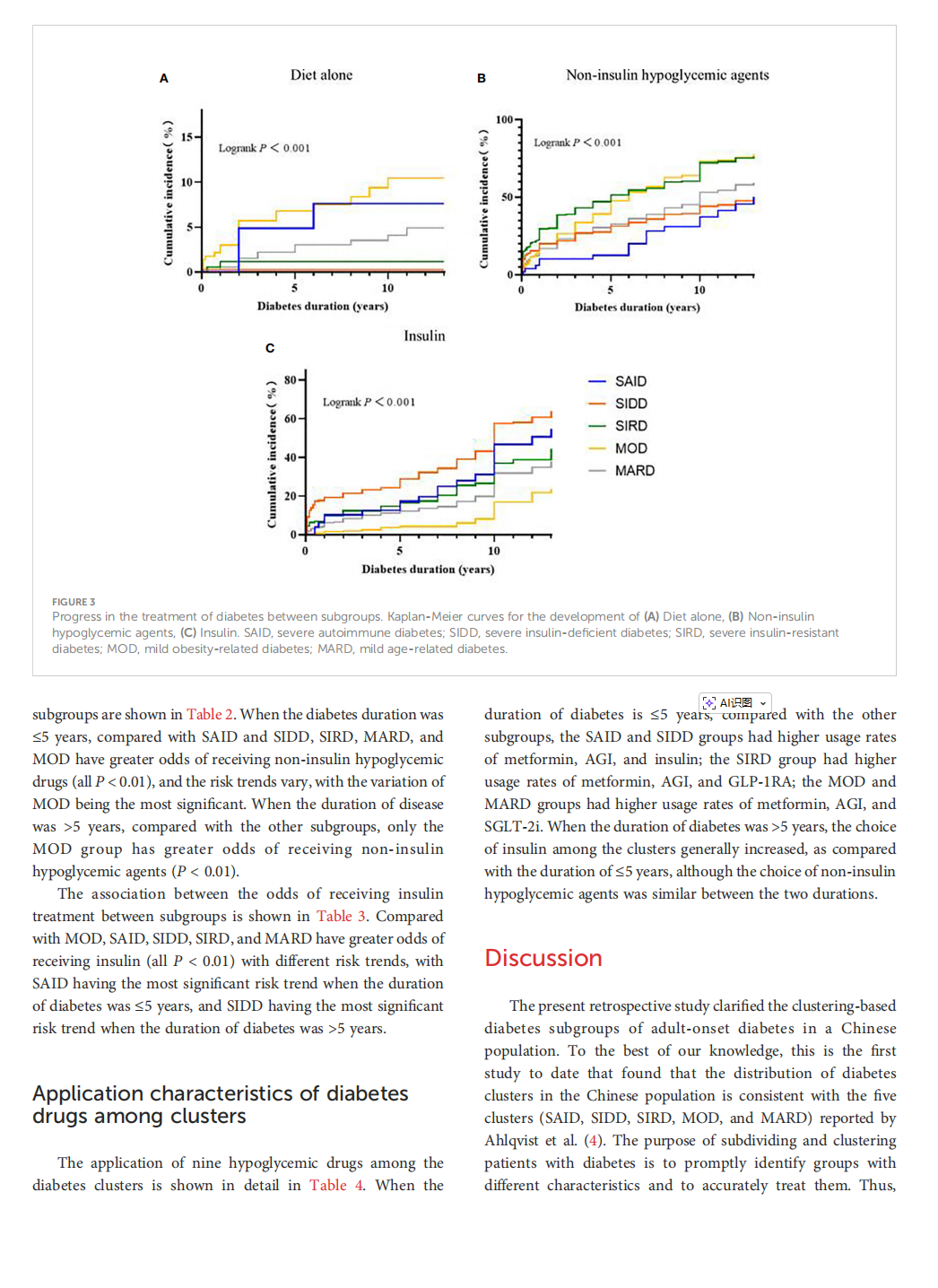
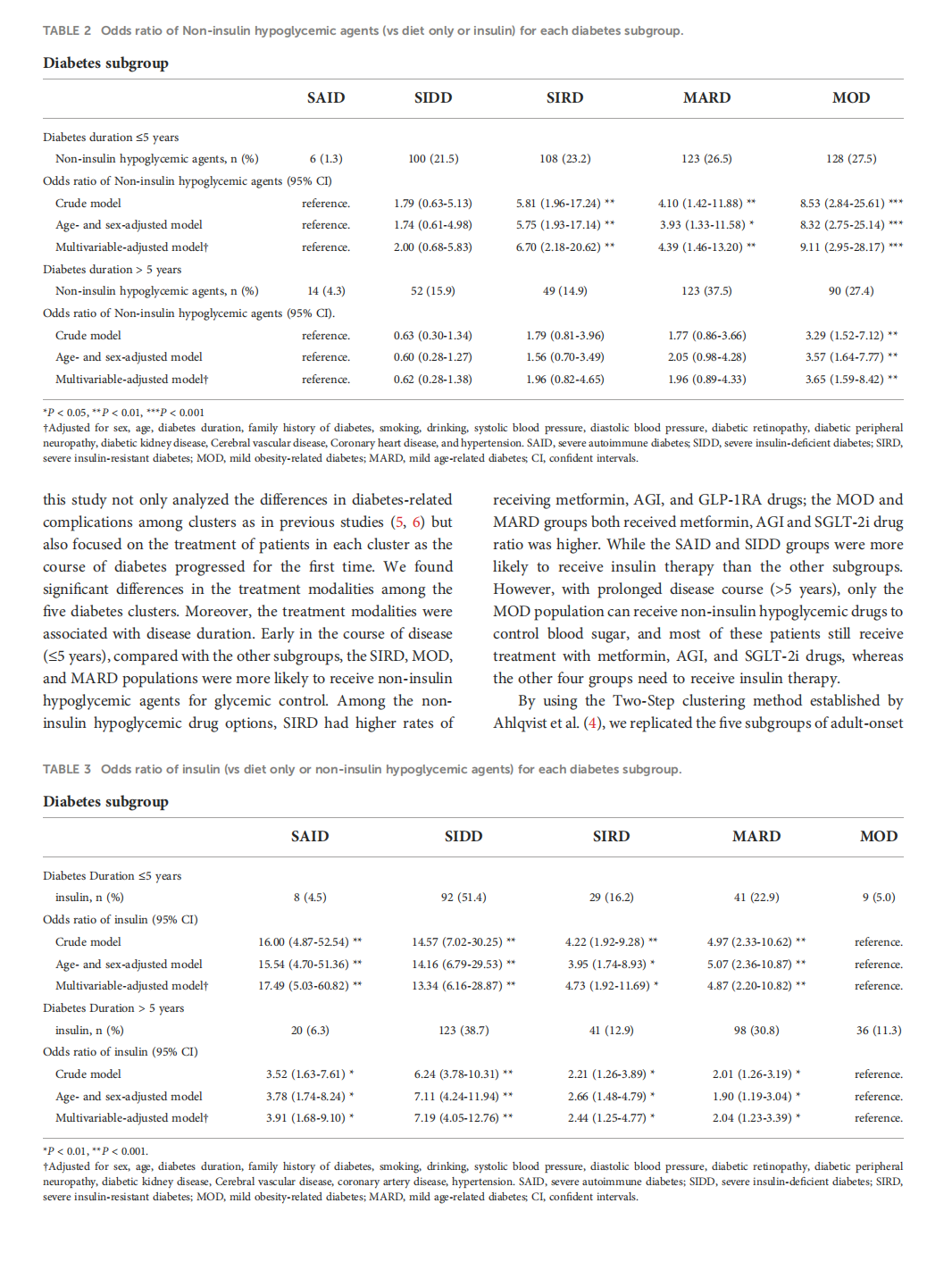
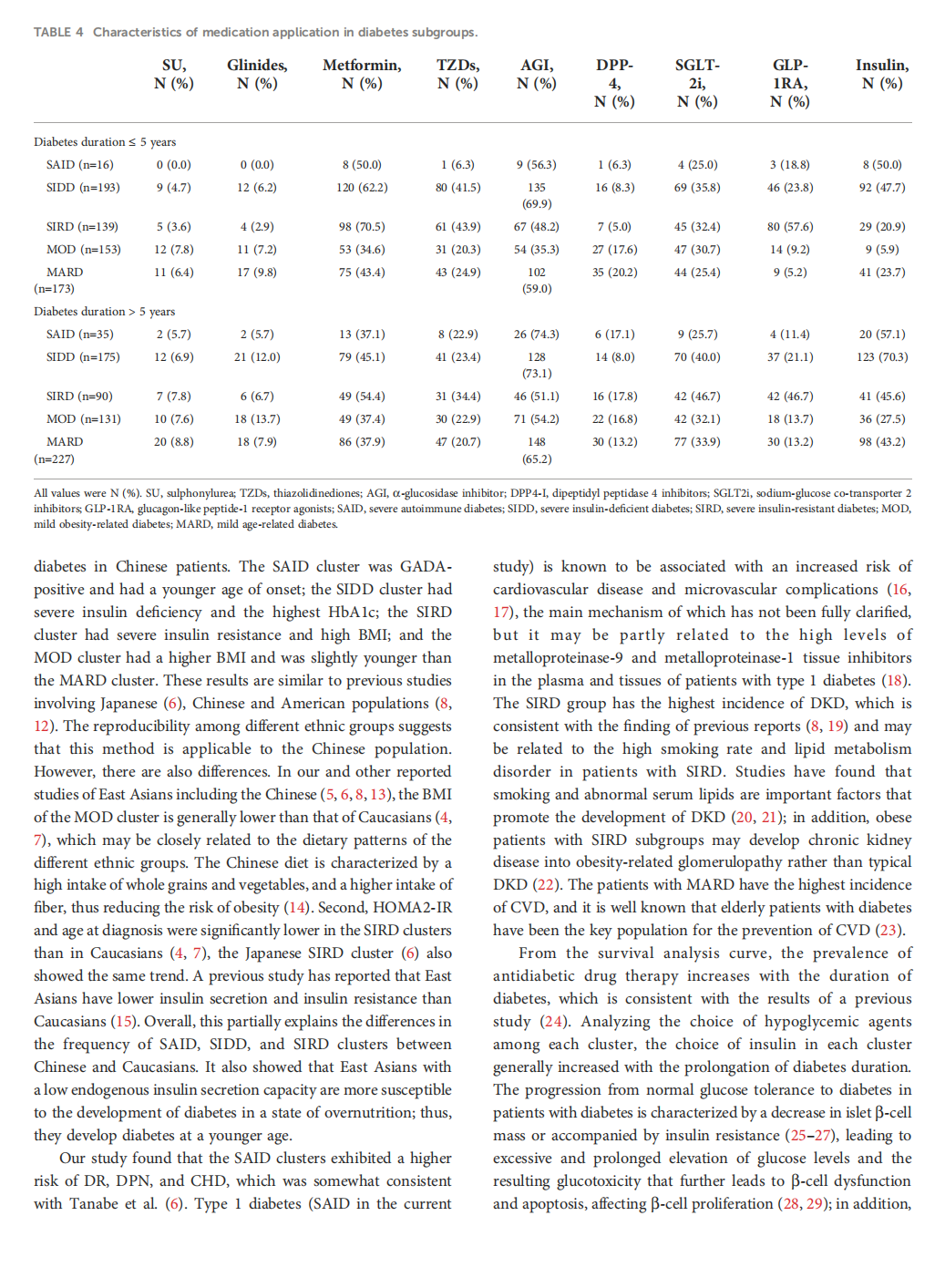



This article is excerpted from the 《 Frontiers in Endocrinology》 by Wound World

- 星期五, 27 2月 2026
The mediating role of diabetes stigma and self-efficacy in relieving diabetes distress among patients with type 2 diabetes mellitus: a multicenter cross-sectional study




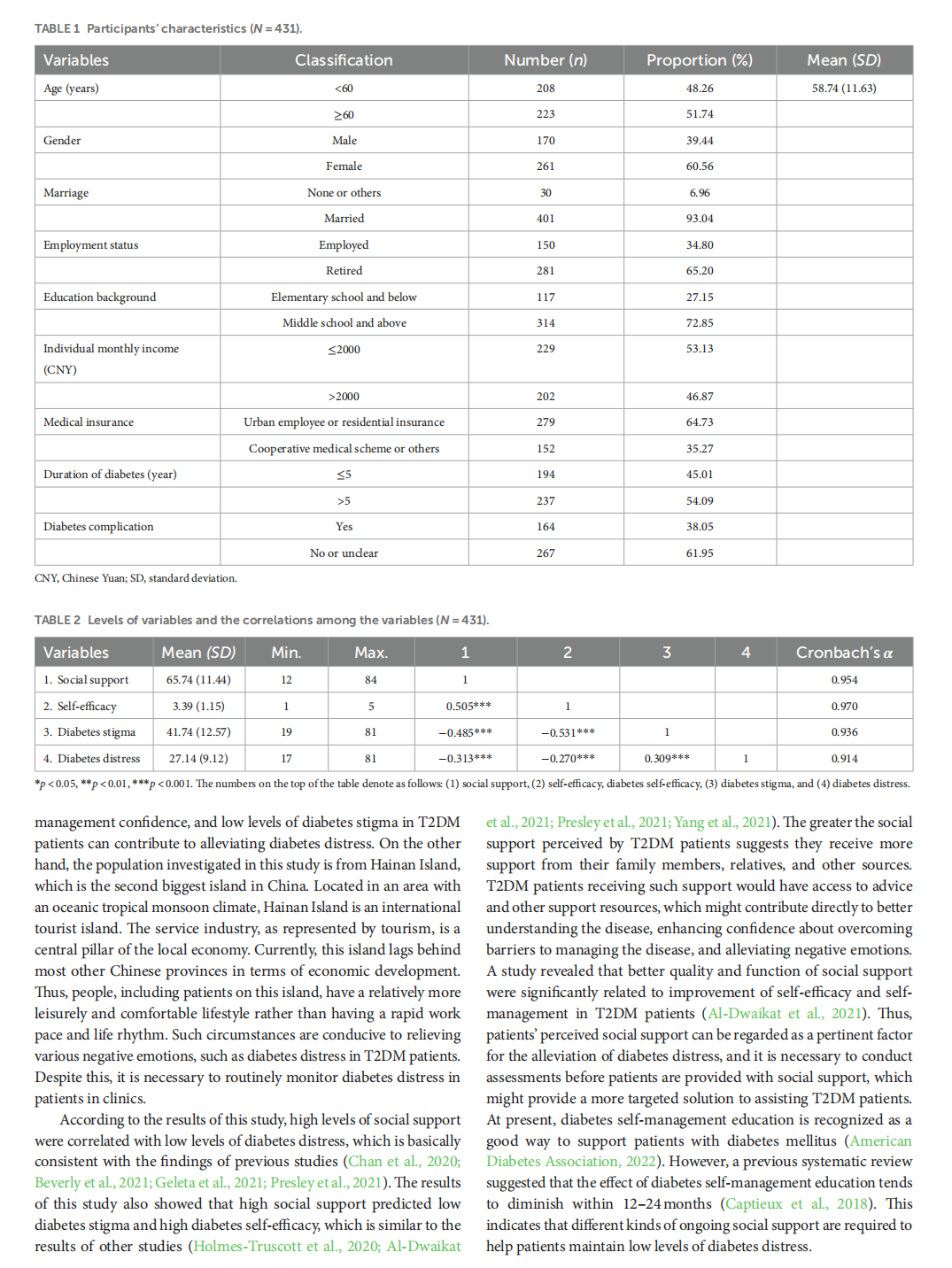



This article is excerpted from the 《Frontiers in Psychology》 by Wound World



