Irrigation of wounds has been shown to aid in removal of wound and dressing debris. Saline cleansing of pressure injury was recommended in 1994 when the first Agency for Health Care Policy and Research (AHCPR) guidelines on treatment were published (Bergstrom et al, 1994). The recommended method used a 35ml syringe and an angiocath to get adequate pressure to lift debris. Saline was advised because it was not toxic to fibroblasts. Of course, in this era of wound care science, the delirious effects of biofilm were not understood.
When the 35ml syringe was no longer stocked in facilities, the required pressure could not be generated. Many wounds were cleansed with 5ml syringes of saline used for endotracheal tube irrigation. Sprinkling normal saline on the wound bed moistened it but did little to remove debris or address the biofilm. As biofilm was increasingly understood to be a healing deterrent, other methods of wound care came into play. Topical dressings with antiseptics were important but wound irrigation was only a minor part of wound care.
Clinically, when wounds became odorous, an old method of topical irrigation and dressings came back into practice. Once only collecting dust on the supply shelves, sodium hypochlorite (commonly known as Dakin’s solution) was back and practitioners were using it often — as a cleanser, an irrigant and as a packing.
Fortunately, the science of wound care is continuing to develop and the evidence on the value of wound cleansing/irrigation is again appreciated. These 10 top tips will review the current state of the science in wound bed preparation, including the detriments to healing when the wound bed is alkalotic and the benefits of an acidic irrigant to promote a wound-healing environment.
1. Protect the acid mantle of the skin by using materials that are at a pH of 5.5
The human epidermis exists at a pH of 5.5. This acid mantle is created by the sebum and sweat glands, and retards growth of pathogenic bacteria as it maintains a normal biome. An acidic pH in the skin keeps the resident bacteria attached to the epidermis. An alkaline environment on the skin disperses the normal skin biome and promotes growth of pathogenic bacteria. Pathogenic bacteria colonise and proliferate in alkaline environments. We often take the skin for granted and use products on the skin that are more alkaline to clean it. Bar soaps tend to be alkaline and lead to irritation and dry skin. The epidermis turns over every 45–60 days in adults. However, in older patients, skin cell turnover is slower, up to 90 days. Therefore, protecting the skin with pH-balanced products is important.
2. Incisions begin healing in an acidic
When an incision is made, the tissue at the site must rely on anaerobic metabolism because of the loss of arterial blood supply. This transitory state is helpful because angiogenesis is best in a slightly acidic environment. In the inflammatory phase, white blood cells within the wound bed generate reactive oxygen species that further lower the pH to 5. Once healing is complete, the wound matrix pH raises to 7, which facilitates collagen synthesis. As the incision normally heals and epidermis appears, the acidic state of the skin reappears.
3. Weigh patients routinely.
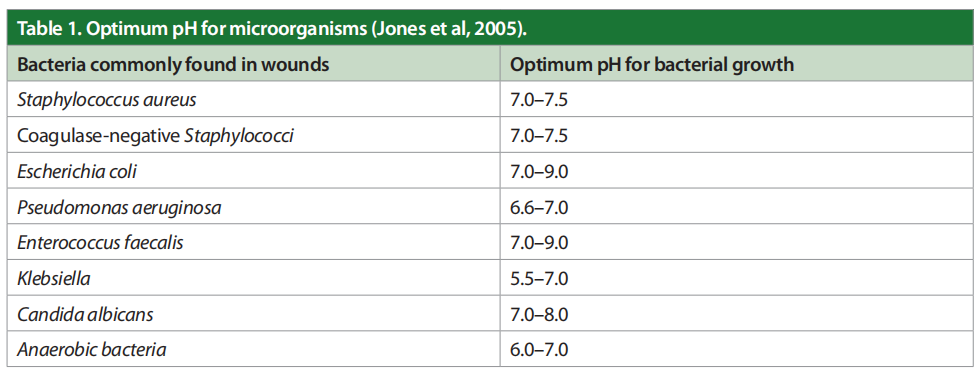
Most chronic wounds get stuck in a very prolonged inflammatory phase due to a persistently elevated bioburden that includes multiple types of planktonic bacteria and their corresponding biofilm. As chronic wounds fail to heal, there is a significant decrease in the wound pH. These organisms drive the pH of the wound bed up, over 8.5. The toxicity of bacterial end products, such as ammonia, increase macrophage activity, control of enzyme activity, slow angiogenesis and create abnormal collagen in the ulcer bed. In brief, wound pH and healing are related. If the acute wound fails to heal, the wound bed becomes alkalotic and colonised with bacteria. The optimum pH for microorganisms is shown in Table 1 (Jones et al, 2005).
4. Hypochlorous acid, sodium hypochlorite and polyhexamethylene biguanide (PHMB) are not the same.
The pH of stabilised hypochlorous acid is 5. It is a weak acid that forms when chlorine dissolves in water and itself partially dissociates, forming hypochlorite. In the white blood cell, hypochlorous acid is generated and destroys bacteria. The pH of sodium hypochlorite solutions (Dakin’s) ranges from 9–10. Dakin’s solution (sodium hypochlorite) was first used to treat shrapnel injuries in World War I and saved countless lives and limbs. Dakin’s solution remained the prevailing method for treating contaminated wounds until the introduction of antibiotics during World War II (McCullough and Carlson, 2015). Then with the emergence of antibiotic resistant organisms in the 1980s, especially in chronic wounds, interest in antiseptic treatment of wounds resurfaced. However, an animal model examined kill rates of common organisms leading to infection. Sodium hypochlorite at 0.0025% and 0.025% did not reduce bioburden from S. aureus or P. aeruginosa (Mangum et al, 2018). Polyhexamethylene biguanide (PHMB) is a strong base, which is highly positively charged at physiological pH. Conflicting evidence exists regarding the activity of PHMB against S. aureus biofilms (Ortega-Pena et al, 2017).
5. Cleanse wounds with mildly acidic solutions
When the focus shifted from “prevention of infection” to “creation of an optimal environment for the repair process”, there was a greater concern about not harming the wound bed. A laboratory study by Wang and associates tested the time to kill Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus when using hypochlorous acid, sodium hypochlorite and hydrogen peroxide (Wang et al, 2007). Table 2 shows the kill times for each organism with each solution. Hypochlorous acid was the fastest agent, killing all three pathogens in under 1 minute.
6. Choose solutions that have low cytotoxicity.
Cytotoxicity is the loss of functional wound healing cells in the wound bed. A common measure of cytotoxicity is to measure the fibroblast cell viability that remains after use of wound cleanser/antiseptics. Most of the cytotoxicity work is conducted in a laboratory. A comparison of viability of fibroblasts when exposed to 1%, 5% and 10% sodium hypochlorite, hypochlorous acid, modified diallyl disulfide-oxide and super-oxidation solutions was completed by Ortego-Pena and colleagues (2017). Findings included that the use of sodium hypochlorous acid had the lowest cytotoxicity, with 75–80% of the cells remaining viable.
Normal saline has no cytotoxicity. However, it provides no antiseptic properties. A clinical study by Wilson and associates measured the rate of healing in venous leg ulcers treated with a solution at pH of 6 compared to a solution at pH of 7.4. The acid solution group healed on average of 16.3sq/ mm/day in comparison to the control group who healed more slowly at 6.3 sq/mm/day. The difference was statistically significant ( p<0.005) (Wilson et al, 1979).
Hydrogen peroxide not only fails to kill organisms; it stimulates osteoclastic bone resorption (Cicek and Cakmak, 2018).
7. Choose a wound cleanser/irrigant that retards biofilms.
Biofilms are matrix-enclosed microorganisms, which can significantly contribute to the pathology of chronic wounds by stimulating inflammation and they cannot be penetrated by systemic antibiotics. Biofilm is influenced by ambient pH. In alkaline wounds, Staphylococcus aureus has enhanced adherence to the wound bed. Pseudomonas and Klebsiella increased production of biofilm in wounds with increased pH (Rippke et al, 2018; Hostacka et al, 2010; Alves et al, 2021).
Topical antiseptics are widely used for wound treatment, with the goal of disrupting biofilm capacity. Harriott and colleagues compared the antimicrobial properties of three commercial wound care solutions on 24-hour-old biofilms (Vashe [HOCl-based]; PhaseOne [HOCl-based]; Sulfamylon [mafenide acetate] for their in vitro activity against bacterial and fungal biofilms. Vashe and PhaseOne displayed excellent bactericidal and fungicidal activity, whereas Sulfamylon demonstrated minimal activity against the biofilms tested. With the exception of Candida albicans, all biofilms were eliminated at either 1 or 10 minutes using Vashe and PhaseOne solutions. In most cases, mafenide was unable to eliminate both bacterial and fungal biofilms, even with 24 hours of treatment. The study indicates that most antiseptics remain effective long enough to prevent biofilm formation; thus, even brief application of an antiseptic agent during initial wound treatment can lead to better wound management outcomes (Harriott et al, 2019).
8. Do not sprinkle wound cleansers on the wound
The purpose of wound cleanser is to separate the debris and biofilm on the wound bed. The solution should be placed on a gauze that is separated into a single layer. The moistened gauze can be placed on the wound itself and allowed to remain in place for 10 minutes. For some patients, this means that the practitioner will need to hold the dressings in place on the wound. Additional assessments, especially those which address adherence to the plan of care can be done during this time.
9. Clean the wound after 10 minutes
The loose debris and dead pathogens should be removed from the wound bed by scrubbing the wound with the gauze used for cleansing. Some clinicians state “clean it like you mean it” to describe this phase.
10. Reduce the risk of periwound damage.
When using wet packing, be certain that the dressings are not oversaturated when placed on the patient. The fluid will run out of the dressing and onto the skin of the patient. If the solution is caustic, the patient’s skin can be burned.
Conclusion
Wound bed pH plays a very important part in wound healing. Alkaline pH favours attachment and growth of pathogens and formation of biofilms. Due to the deleterious effect of a high tissue bacterial burden on the process of wound healing, an effectual antimicrobial agent becomes a therapeutic imperative. Targeting the wound pH with mildly acidic wound cleansing will aid healing.
References
1. Alves PJ, Barreto RT, Barrios BM et al (2021) Update on the role of antiseptics in the management of chronic wounds with critical colonization and/or biofilm. Int Wound J 18(3): 342–58
2. Bergstrom N, Bennett MA, Carlson CE et al (1994) Treatment of Pressure Ulcers. Clinical Practice Guideline, No. 15. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, Agency for Health Care Policy and Research. AHCPR Publication No. 95-0652
3. Cicek, E, Cakmak E (2018) Hydrogen peroxide induced oxidative damage on mineral density and mechanical properties of bone. Braz Arch Biol Technol 61 [online edition]
4. Harriott MM, Bhindi N, Kassis S at el (2019) Comparative antimicrobial activity of commercial wound-care solutions on bacterial and fungal biofilms. Ann Plast Surg 83(4): 404–10
5. Hostacka A, Ciznar, I Stefkovicova M (2010) Temperature and pH affect the production of bacterial biofilm. Folia Microbiol (Praha) 55(1): 75–8
6. Jones EM et al (2015) The effect of pH on the extracellular matrix and biofilms. Adv Wound Care (New Rochelle) 4(7): 431–9
7. Mangum LC, Franklin NA, Garcia GR et al (2018) Rapid degradation and non-selectivity of Dakin’s solution prevents effectiveness in contaminated musculoskeletal wound models. Injury 49(10): 1763–73 McCullough M, Carlson GW (2014) Dakin’s solution: historical perspective and current practice. Ann Plast Surg 73(3): 254–6
8. Ortega-Peña S, Hidalgo-González C, Robson MC, Krötzsch E (2017) In vitro microbicidal and biofilm and cytotoxic effects of different commercial antiseptics. Int Wound J 14(3): 470–9
9. Rippke F, Berardesca E, Weber TM (2018) pH and microbial infections. Curr Probl Dermatol 54: 87–94
10. Wang L, Bassiri M, Najafi R et al (2007) Hypochlorous acid as a potential wound care agent: part 1. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds 6: e5 Wilson IA, Henry M, Quill RD, Byrne PJ (1979) The pH of varicose ulcer surfaces and its relationship to healing. Vasa 8(4): 339–42
This article is excerpted from the Wounds International 2022 | Vol 13 Issue 2 by Wound World.


