Medical device-related pressure ulcers (MDRPUs) are a type of pressure ulcer (PU, also known as a pressure injury) caused by sustained forces applied by skin contacting medical devices (Gefen et al, 2020, 2021; Lustig et al, 2021). These injuries occur frequently in acute care settings and, while their incidence and prevalence vary and depend on the specific type of the hospital setting and the quality of practice, pooled data point to high occurrence rates, 12% and 10%, respectively (Jackson et al, 2019). The impact of MDRPUs is multifaceted. The injury may become hard-to heal, as the affected patients often belong to high-risk groups with fragile skin. These wounds most often occur on the face, in association with respiratory equipment, such as non-invasive ventilation masks (Jackson et al, 2019; Gefen et al, 2020; Dang et al, 2021), and could lead to permanent scarring that may reflect on the body image perception at all ages and throughout life. From a financial perspective, MDRPUs not only add to the direct treatment (material and labour) costs, but may also provoke litigation acts, being a hospital-acquired injury in the vast majority of cases.
Cells in the soft tissues under or near a medical device that is in close contact with the skin undergo extreme shape distortions that result from the forces applied by the device, and which ultimately lead to the loss of biological function of the deformed cells (Gefen et al, 2020, 2021; Lustig et al, 2021). Cells and the tissues they reside in are, in essence, physical structures that contain structural elements made of proteins, with specific mechanical roles of resisting or supporting forces through tolerable deformations. If the force magnitude or exposure time exceeds a critical threshold (the threshold level being unique to the cell type and tissues of the individual), then, as with any physical structure, the cells and tissues would be damaged, and cells will eventually die.
The direct and primary cause of the cell death associated with MDRPUs is deformation-inflicted damage where the plasma membrane, which is the wall surrounding the cell and protecting its organelles, does not receive sufficient structural support from a dysfunctional cell skeleton (cytoskeleton), after the mechanical failure of too many cytoskeletal fibres under the sustained loading. Once a cell becomes unable to maintain the integrity of its plasma membrane, pores appear along the plasma membrane, through which molecules and ions may cross without active regulation. The uncontrolled molecular traffic destroys the delicate state of steady internal, physical, and chemical balances maintained by living cells, known as homeostasis, which rapidly leads to apoptotic cell death (Lustig et al, 2021).
Consequently to the deformation-induced cell death, localised inflammatory oedema develops, as a natural response of the immune system to the initial cell death events (Gefen et al, 2020, 2021; Lustig et al, 2021). In cases where the oedematous soft tissues cannot effectively swell, when they are sandwiched between a rigid bony surface and a stiff device, the interstitial tissue pressures rise sharply. The resulting build-up of high interstitial pressures in the oedematous tissues further deforms the cells within, causing a secondary, inflammatory cell damage, through additional loss of cytoskeletal integrity, more poration of the plasma membranes and wider spread death of cells (Gefen et al, 2020, 2021; Lustig et al, 2021). As this damage process escalates, and without relief or repositioning of the medical device, the localised rise in interstitial pressures may eventually cause obstruction or even partial occlusion of the vasculature or lymphatic networks under or near the applied device. This adds hypoxia and acidosis to the already stressed cell environment, thereby causing tertiary ischaemic damage. A MDRPU caused by a ventilation mask is a classic example of this vicious cycle of injury (Gefen, 2021). As the mask directly compresses and shears a heated, and thereby moist, facial skin against the rigid skull surface (and even more so, when the straps are overtightened), extreme mechanical stress concentrations develop in the affected soft tissues. Facial tissues under a ventilation mask have limited space for swelling when the oedema progresses, particularly over the nasal bridge and cheek (zygomatic) bones in this configuration, so it is not surprising that these anatomical sites are the ones that are most commonly injured; 10–33% of the patients among those who use a ventilation mask suffer a mask-related facial injury within just several hours after applying the mask (Otero et al, 2017; Brill et al, 2018; Walker et al, 2019; Gefen, 2021).
This susceptibility of facial skin to MDRPUs is further amplified by the forces of mounting and tightening the mask to the head, and the extreme microclimate conditions which expose facial skin under and near the mask to almost 100% humidity (Yamaguti et al, 2014; Otero et al, 2017; Alqahtani et al, 2018; Brill et al, 2018).
To protect the face from injury, healthcare professionals who are aware of the implications of attaching devices, such as ventilation masks, to the skin for hours, apply protective dressings to the at-risk facial sites. Currently, there is no definitive or mandatory guidance with regards to the type of cushioning and padding materials for achieving optimal skin and subdermal tissue protection, although the International Pressure Ulcer/Injury Prevention and Treatment 2019 Guideline recommends using foam dressings, for which the majority of clinical evidence exists (Walker et al, 2019). In addition, there are no commercial products with a specific indication for medical device related pressure ulcer prophylaxis (MDRPUP).
The clinical practice of facial protection is, therefore, based on cutting available wound dressing materials (that is, treatment dressing products) to desired sizes and shapes, e.g. to cushion the nasal bridge or cheeks from a ventilation mask. Real-world choices of the specific wound dressing type for this practice are based on considerations such as subjective clinical judgment and experience, intuition and sometimes improvisation, availability of the dressings at the facility, the cost of the available dressing types, previous training and the local commonly accepted practice.
Currently, the most commonly used materials for facial MDRPUP are hydrocolloid dressings (HCDs) and foam dressings (FDs) (Moore et al, 2018; Cai et al, 2019). The former type of dressing typically contains a dispersion of gelatine, pectin and carboxy‐methylcellulose together with other polymers and adhesives which form a flexible wafer. The latter dressing type is made of open cell, hydrophobic, polyurethane foam sheets (Moore et al, 2018). Often, nurses who choose one type over the other are unaware of the underlying differences in material behaviours and the biomechanical considerations and implications of their selection, in particular concerning the compatibility of these dressing types with skin.
Accordingly, this article aims to compare the suitability of HCDs versus FDs for the specific purpose of facial MDRPUP, based on biomechanical considerations which are explained here in language that is familiar to healthcare professionals.
Hydrocolloid versus foam dressing materials for prophylactic use
Hydrocolloid materials are a popular option among clinicians for protecting facial skin from MDRPUs (Cai et al, 2019), despite that HCDs have been reported to sometimes cause inflammatory skin irritations, both in animal models and the clinical setting (Omura et al, 2010; Yamane et al, 2015). When compared to controls who received no skin protection, prophylaxis by means of HCDs was reported to be effective over the nasal bridge (Bishopp et al, 2019), but there is a paucity of information concerning the protective efficacy of HCDs with respect to alternative dressing materials for prophylaxis. In this context, a fundamental doubt concerning the application of HCDs for MDRPUP is their high absorption capacity which typically causes HCDs to dilate in the presence of moisture, resulting in the development of swelling forces (Ferrari et al, 1994; 1995; Lanel et al., 1997). These swelling forces may gradually distort the skin under the HCD cuts, cause shearing of the skin and, overall, increase the level of mechanical stresses on facial skin and underlying tissues. Indeed, several clinical studies have questioned the effectiveness of HCDs in PU and MDRPU prevention. For example, Hao and colleagues (2015) indicated that HCDs did not reduce erythema in acute care patients who were at-risk for PUs. Compared with HCDs, soft foams have the potential to be better suited to the functions of MDRPUP. Specifically, unlike HCDs which swell rapidly and considerably upon contact with moisture, foams swell substantially less, and in fact, are utilised to dampen the swelling effect induced by moisture in multi-material dressings (Lim & Lee, 2003; Lin et al., 2015; Jin et al., 2016). Moreover, the dehydration of saturated HCDs was reported to decrease dramatically after 30 minutes with respect to the steady dehydration from polyurethane FDs (Hasatsri et al., 2018), rendering HCDs more likely to cause damage (skin maceration) when used prophylactically in a humid environment such as under ventilation masks. Nevertheless, the most profound difference between HCDs and FDs is in their compressive stiffness properties, as discussed next.
Considering the dressing-skin biomechanical compatibility: The importance of the stiffness matching criterion for effective prophylaxis
The biomechanical compatibility of any padding or cushioning materials (or multi material prophylactic dressings) with skin can be assessed in terms of the mechanical stiffness matching between the skin and the material or structure applied for tissue protection (Bader et al., 2019; Gefen et al., 2020). This is based on the fundamental mechanical engineering principle that, in interfacing objects, material stiffness gradients would always cause mechanical stress concentrations and greater deformations in the softer element. In the context of MDRPUP, a dressing that is considerably stiffer than skin will provide poor prophylactic performance, because the large difference in stiffnesses of the dressing versus the skin will promote high tissue distortions under and particularly, at the perimeter or borders of the applied (stiff) dressing [Figure 1].
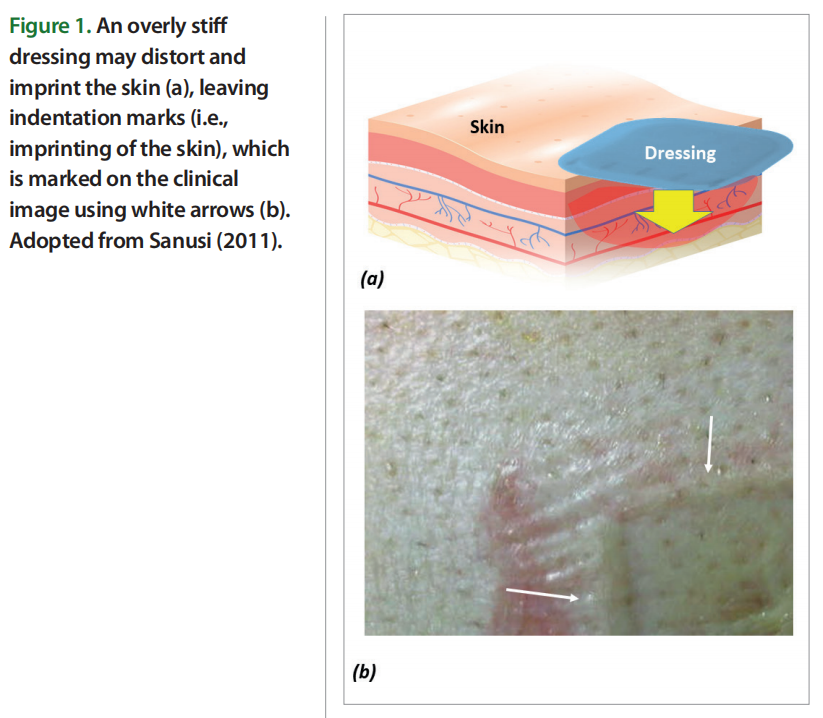
Of note, in MDRPUP, the compressive stiffness of a dressing and the compressive stiffness of the skin region covered by the dressing are the most important and relevant properties to consider, given the common techniques of device attachment to skin which apply localised, intense compressive forces to the skin, such as for ventilation masks that are strapped to the head. This stands in contrast to the requirements for dressings used to prevent bodyweight force induced PUs, such as at the sacrum and heels, where other biomechanical considerations apply, that account for the potential occurrence of substantial tension and shearing of the skin due to the bodyweight forces.
For example, a hypothetical dressing made of steel and applied for MDRPUP would indent and imprint the skin and intensively shear it along its boundaries, which is why excessively stiff dressings acting as ‘shields’ are a poor solution for preventing MDRPUs (Schwartz and Gefen, 2019; Gefen et al, 2019). Based on the above concept, one could theoretically argue that the ideal preventative dressing should be made of thick (living) skin, which, by definition, will not have a stiffness gradient with (the other) skin, but of course, this is not feasible in the real world. The best that material and biomedical engineers can do is to approach the stiffness of native skin as closely as possible using synthetic or processed biological materials that are biocompatible, and have a compressive stiffness matching ratio (CSMR) E/Eskin which approaches unity (i.e., CSMR→ 1), where E is the elastic modulus — a stiffness measure of the candidate protective material, and Eskin is the elastic modulus of native, living human skin [Figure 2].
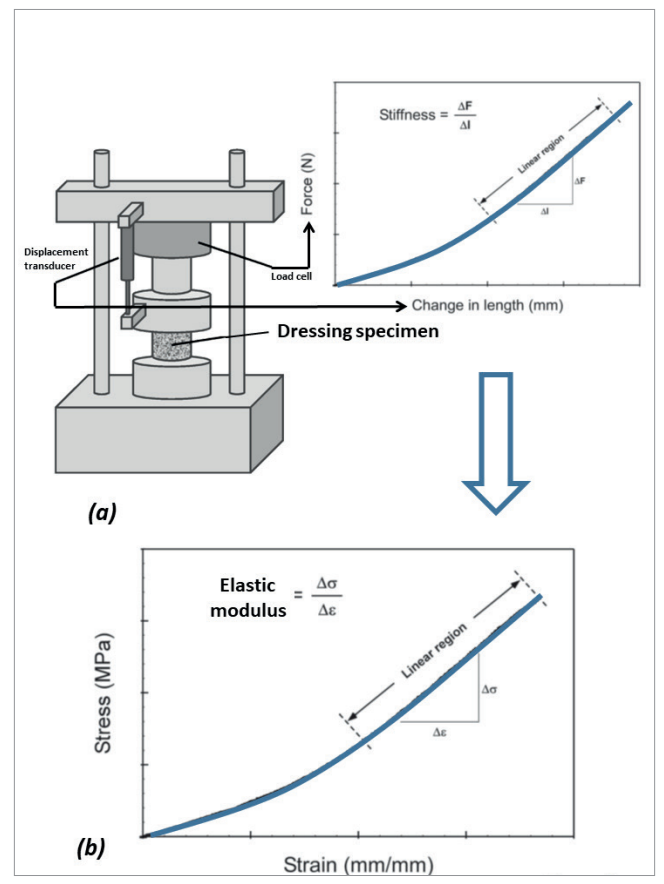
Figure 2. The concepts of measuring the compressive stiffness of dressing materials using a material testing system: (a) A force displacement curve is acquired by measuring the force required to compress the dressing specimen under an incrementally increasing displacement load. The force is continuously measured by means of a load cell, and likewise, the displacement is recorded from a displacement transducer, both are integrated in the materials testing machine; (b) The measured force values are converted to stresses (σ), through dividing the force level by the initial (undeformed) cross-sectional area of the dressing specimen. Concurrently, the measured displacement values are converted to strains (ε), through dividing the displacement data by the initial specimen height. Finally, the compressive elastic modulus of the dressing specimen is calculated, as the slope of the linear portion of the stress-strain curve, Δ σ/Δ ε which makes a material stiffness measure. The lower the compressive forces that would require to induce a certain displacement magnitude of a tested dressing specimen, the less stiffness (i.e., lower elastic modulus) that the dressing would exhibit.
Specifically, the elastic modulus property is a commonly used quantitative engineering stiffness measure for materials, defined as the ratio of the mechanical force exerted upon a substance to the resultant deformation of that material [Figure 2]. The elastic modulus of a material is typically associated with the direction of the load applied in the laboratory setting (also known as the loading mode), which may be tension, shear or compression, the latter being the most relevant primary loading mode in the context of MDRPUP [Figure 2]. The E/Eskin property ratio for the compressive elastic moduli is, hence, a quantitative performance measure characterising the potential of a candidate material structure in delivering effective protection to skin. Such objective quantitative approaches employing fundamental bioengineering principles have not been applied sufficiently in the context of product selection for MDRPUP, and too often, unfortunately, false premises based on clinical intuition and subjective opinions overweigh basic engineering science considerations. The case of HCDs versus FDs for MDRPUP makes a good example of where clinical intuition does not always work, as further discussed below.
The stiffness matching ratios for hydrocolloid versus foam dressings
Compressive elastic modulus measurements of commercial wound dressing products are regularly conducted at the laboratory of the author. Example and representative test data for a market-dominant HCD commonly used for MDRPUP in clinical practice are shown in Figure 3. To measure the compressive elastic behaviour and properties of these dry and moist HCDs, considering their potential viscoelastic (solid-fluid) behaviour through characterisation of short-term (also known as ‘instantaneous’) and long-term elastic modulus properties, by means of uniaxial unconfined compression testing, a test protocol was developed, based on the ASTM Standard Test Methods for Rubber Properties in Compression D575 – 91 (2018), which was modified as follows. A load cell with a nominal range 2-2kN ± 0.5% of accuracy, connected to a material testing machine (Instron Corp., model 5944, Norwood, MA, USA) operating with BlueHill® software (Instron Corp.) was used to record the applied compressive loads. These loads were applied via flat compression anvils which were made larger than the dressing specimen surface area. The compressive forces were applied and removed in three successive cycles. The first two cycles were of a ramp-and-release form, for the purpose of preconditioning the dressing specimens. The third cycle was of a ramp-and-hold form, and was aimed for acquisition of the force data towards calculation of the instantaneous and long-term compressive elastic modulus properties of the studied HCD. After measuring the thickness of each dressing test specimen using a calliper, the dressing specimens were piled together to an overall stack thickness of approximately 11mm and placed between the platens of the material testing machine. Thin sheets of sandpaper were then placed between the uppermost dressing specimen surface and the superior testing machine platen, and likewise, between the lowest dressing specimen surface and the inferior machine platen, to avoid potential frictional sliding movements of the compressed dressing specimens. Force was applied at a displacement rate of 12mm/ min, up to a compressive strain of 50%, after which the force was immediately released at the same (unloading) rate. This loading/ unloading cycle was repeated a second time (for preconditioning), following which the dressing specimens were considered as preconditioned. Then, the force was applied for the third time, until a strain of 50% was again reached [Figure 3a], and this strain level was held for a period of 320 seconds, during which the displacement was maintained so that the dressing specimens remained compressed. The force data measured instantaneously after the third-ramp phase of the loading cycle and every 0.1 seconds after completion of the ramp phase (until changes in the measured force became less than 1%) were acquired, to form a force-relaxation curve [Figure 3b]. Calculation of the target compressive strain (50%) was based on the undeformed thickness of each dressing specimen (acquired prior to the first force application).
The same procedure described above was repeated to measure the elastic moduli of moist HCDs, as follows. An isotonic saline solution was prepared by dissolving 9 g of NaCl in 1 litre of distilled water, boiling the solution for 15 minutes and then cooling it to room temperature. This saline solution was then uniformly sprayed onto a flat semipermeable surface comprised of a 0.8 ± 0.1mm-thick dense chamois cloth, thereby simulating sweaty moist skin (Montain et al, 2007; Cui and Schlessinger, 2015; Dąbrowska et al, 2016). Dressing specimens, cut to form standard circular test specimens, were placed on the moist fabric. To verify that the moisture penetrated the depth of the dressing material, in the specific context of MDRPUs caused by ventilation masks, where it is not only the skin which perspires, but also, near 100% humidity exists in the space of the mask, the dressing specimens were placed with their semi-permeable, medical device-facing aspect on the moist fabric to allow them to absorb the moisture. Next, the dressing specimens were loaded with a 390g weight, distributed over an area of 200cm2 to simulate a sustained pressure of 195 Pa from a mask (Peko Cohen et al, 2019). The dressing specimens were left between the weight and the moist cloth for approximately 7 hours (i.e., overnight) (Alviset et al, 2020; Brusasco et al, 2021), after hermetically covering the setup that included the weight and the moistened dressing specimens on the chamois fabric, to avoid evaporation effects. Considering that the maximum sweat rate for healthy adults is approximately 1mg/min cm2 (Jessen, 2020), and, since the effective moist cloth area was 200cm2 , a uniform layer of 40ml of saline solution was applied to the chamois cloth prior to applying the weight, as described above. We considered this volume of saline spray to be effective for maintaining the dressings in a constantly humid environment, which simulated the combined influence of mild to moderate perspiration, plus the moisture from the humid air in the space of the mask. After the 7-hour moisture treatment, the HCD specimens were released from the weights. Finally, the instantaneous and long-term elastic moduli of the moist dressing specimens were also measured, in addition to the corresponding dry properties [Figure 3c].
It was shown that the dry HCD specimens were mildly stiffer than the moist ones (~1.2-times and ~1.1-times for the instantaneous and long-term elastic moduli, respectively; Figure 3c). In addition, it was observed that the HCD specimens underwent substantial stress relaxation [Figure 3b], such that the instantaneous elastic moduli were 2.2-fold and 2-fold greater than the long-term moduli for the dry and moist specimens, respectively, which indicates a strong viscoelastic behaviour of this dressing material which could be expected, given its moisture absorbency. Overall, given the relatively short viscoelastic relaxation times (~5 minutes) with respect to the typical timeframes for development of MDRPUs, it appears, based on the current test data [Figure 3b,c], that the long-term elastic modulus is the appropriate stiffness characteristic of the HCDs to consider in the context of MDRPUP. This specific property was statistically indistinguishable between the dry and moist test conditions for the particular HCD under investigation [Figure 3c], and hence, averaging this long-term elastic modulus property for maximising the statistical power was appropriate, and yielded a value of 435 kPa. Overall, this value is a conservative measure for the compressive elastic modulus of the tested HCDs.
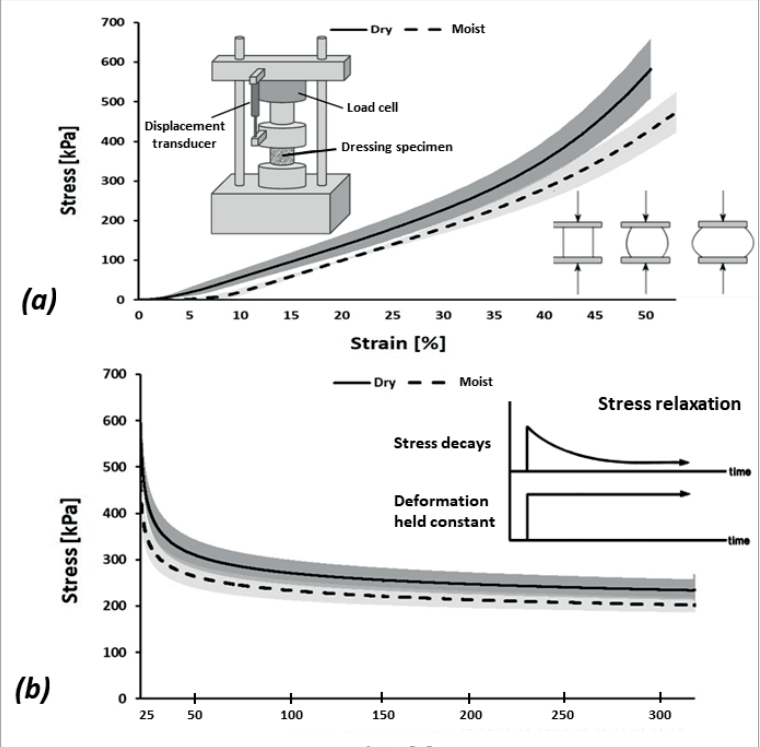
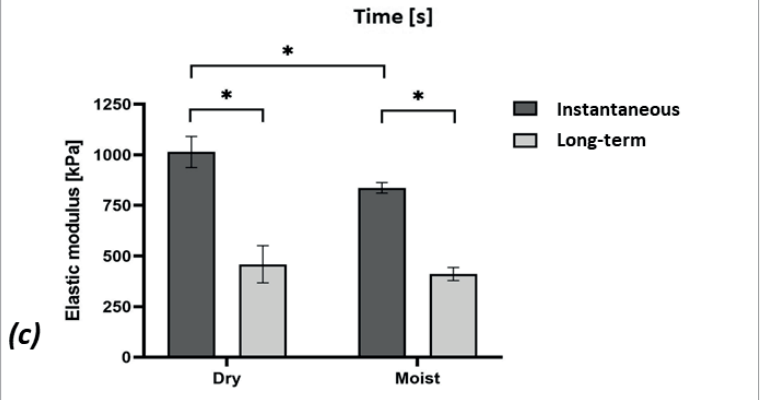
Figure 3. Results of mechanical testing of a market-leading commercial hydrocolloid dressing: (a) Compressive stress-strain test data; (b) Stress relaxation test results, and; (c) The instantaneous and long-term elastic moduli of the hydrocolloid dressing at its dry and moist conditions. The long-term elastic moduli of this hydrocolloid dressing are at least 12-times greater than facial human skin stiffnesses (as detailed in the text).
The above result facilitates evaluation of the extent of the matching between this marketpopular HCD and native adult human skin, i.e., calculation of the CSMR between HCDs and skin. In this context, the literature reports that the in vivo compressive elastic moduli of facial skin, measured through indentation (which is the closest loading mode to the real-world skin-mask interactions) range from 1 to 200 kPa (Zheng and Mak, 1999; Pailler-Mattei et al, 2008; Zahouani et al, 2009; McKee et al, 2011; Flynn et al, 2013; Kalra et al, 2016a; Dai et al, 2019). As noted by McKee and colleagues (2011), the range of stiffnesses reported for human skin studied by means of indentation testing is wide, hence they conducted a statistical outlier analysis of reported empirical data, following which they concluded that the elastic moduli of human skin are within the 6-222 kPa range, averaging at approximately 85 kPa.
Accordingly, the CSMR for HCDs with skin from various anatomical sites is EHCD/Eskin= 435/85 = 5.1, that is, HCDs are more than 5-times stiffer than human skin. Specific analyses for facial regions relevant to prevention of maskrelated MDRPUs can further be made, based on the work of Luboz and colleagues (2014) who evaluated regional facial skin elastic moduli by combining a negative pressurebased experimental technique with computer modelling. They discovered that among 16 young and healthy subjects, the mean elastic moduli of facial skin at the forehead, cheekbones, and lower lips were 17 kPa, 35 kPa and 34 kPa, resulting in corresponding EHCD/ Eskin of 25.6, 12.4 and 12.8. In other words, when targeting the CSMR analyses specifically to facial skin, the EHCD/Eskin is no less than 12.
Noteworthy is the fact that fragile skin, such as that of pre-term babies, infants or elderly, may be substantially less stiff (Kalra et al, 2016b), for example, Boyer et al (2009) reported that the elastic moduli of skin in a group of 51-70 years-old was 33% lower in comparison to a group of 18–30 years-old subjects. Considering a corresponding skin fragility factor (kSFF) of 2/3 for aged and frail skin, the CSMR for HCDs, EHCD/ (kSFF×Eskin), may rise to above 50, indicating that HCDs are not even within the same order of-magnitude of stiffness as fragile skin. This may indeed explain the erythema under HCDs observed in high-risk geriatric patients (> 60 years-old) hospitalised in acute care, as reported by Hao et al (2015).
Contrarily to HCDs, FDs exhibit a substantially lower stiffness. Using experimental procedures similar to the one reported above, we characterised the elastic moduli of foam dressing materials in our published work within the 8–99 kPa range (Levy et al, 2017; Peko Cohen et al, 2019; Orlov and Gefen, 2021), which falls within the above range of stiffnesses of adult skin. Sieracki and colleagues (2020) recently mapped the compressive elastic moduli of commercial FDs by the major global dressing manufacturers, where the range of compressive elastic moduli was, similarly, 7 to 31 kPa. Considering, as an example, the Mepilex® Lite foam dressing (manufactured by Mölnlycke Health Care (Gothenburg, Sweden)), which is a popular FD for MDRPUP, and that was also tested in the laboratory of the author using the same protocol as the HCD referred to above, we found that its long-term elastic modulus in a moist condition is 4.1 kPa (i.e., 106-times less stiff than the tested HCD). In terms of the CSMR of FDs with respect to fragile facial skin, EFD/(kSFF×Eskin), based on the Luboz et al (2014) work, the property ratios are 4.1/(0.67×17)=0.4, 4.1/(0.67×35)=0.2 and 4.1/(0.67×34)=0.2, all reasonably close to unity. In other words, while CSMR values of the HCD with fragile skin were >50, demonstrating extremely poor biomechanical efficacy for facial MDRPUP, the Mepilex Lite data exhibits good stiffness matching, and, thereby, high protective efficacy. The above analyses question the selection of HCDs for MDRPUP, particularly concerning the prevention of injuries associated with ventilation masks applied to fragile facial skin.
Summary and concluding remarks
The alleviation of localised and sustained soft tissue loads is the most fundamental requirement from any type of prophylactic dressing in MDRPUP; avoiding sharp stiffness gradients between the skin and the protecting dressing serves this purpose. The CSMR is an intuitive and easy-to-implement biomechanical performance measure in this regard, and should be reported by manufacturers of dressings intended for MDRPUP. Based on the CSMR criterion described here, HCDs which are popular for facial skin protection from MDRPUs (Moore et al, 2018; Cai et al, 2019), probably due to historical reasons and availability, exhibit poor biomechanical prophylactic efficacy in protecting healthy skin and more so, in preventing injuries in fragile or aged skin, as demonstrated in this article. Foams, on the other hand, have substantially lower stiffness, which is similar to that of living human skin, and, though FDs vary in their compressive stiffnesses (Sieracki et al, 2020), some commercial low stiffness FDs in fact reach CSMR values that approach the ideal unity.
In their work “Tradition, rituals and standards, in a realm of evidenced based nursing care”, Zeitz and McCutcheon (2005) have elegantly described examples of nursing practice that are not evidence-based. They stated that “despite the ongoing work around evidenced-based practice, elements of nursing practice remain based on tradition. Routines and rituals are driving care rather than clinical judgement. The complexities of practice limit the possibilities for change. These complexities include the systems in which nurses practice, the fear of medicolegal repercussions, and the sense of security that rituals provide”.
These authors further explained that elements of clinical practice are often based on tradition and mottos such as ‘the way it has always been done’, and are undertaken as historical routines and rituals, rather than directed by clinical judgement and contemporary knowledge. The application of HCDs for MDRPUP seems to be a characteristic example for such behaviour, as it clashes with contemporary biomechanical scientific knowledge and lacks reasoning. Wound care professionals should therefore adopt objective, standardised, and quantitative research-based approaches in their clinical decision-making processes, to grade and eventually select the optimal dressings for prophylactic applications. The CSMR described here is an example basic bioengineering measure that should be demanded by clinicians and disclosed by manufacturers wherever bestpractice MDRPUP is needed.
References
1. Alqahtani JS, Worsley P, Voegeli D (2018) Effect of humidified noninvasive ventilation on the development of facial skin breakdown. Respir Care 63(9): 1102–10
2. Alviset S, Riller Q, Aboab J et al (2020) Continuous Positive Airway Pressure (CPAP) face-mask ventilation is an easy and cheap option to manage a massive influx of patients presenting acute respiratory failure during the SARS-CoV-2 outbreak: A retrospective cohort study. PLoS One 15(10): e0240645
3. ASTM Standard Test Methods for Rubber Properties in Compression D575 – 91, ASTM International, West Conshohocken, PA, USA, 2018. Available at: https://bit. ly/3rOKr2e (accessed 15.02.2022)
4. Bader DL, Worsley PR, Gefen A (2019) Bioengineering considerations in the prevention of medical devicerelated pressure ulcers. Clin Biomech (Bristol, Avon) 67: 70–7
5. Bishopp A, Oakes A, Antoine-Pitterson P et al (2019) The Preventative Effect of Hydrocolloid Dressings on Nasal Bridge Pressure Ulceration in Acute Non-Invasive ventilation. Ulster Med J 88(1): 17–20
6. Boyer G, Laquièze L, Le Bot A et al (2009) Dynamic indentation on human skin in vivo: ageing effects. Skin Res Technol 15(1): 55–67
7. Brill AK, Pickersgill R, Moghal M et al (2018) Mask pressure effects on the nasal bridge during short-term noninvasive ventilation. ERJ Open Res 4(2): 00168-2017
8. Brusasco C, Corradi F, Di Domenico A et al (2021) Galliera CPAP-Covid-19 study group; collaborators of the Galliera CPAP-COVID-19 study group are. Continuous positive airway pressure in COVID-19 patients with moderate-to-severe respiratory failure. Eur Respir J 57(2): 2002524
9. Cai JY, Zha ML, Chen HL (2019) Use of a Hydrocolloid Dressing in the Prevention of Device-related Pressure Ulcers During Noninvasive Ventilation: A Meta analysis of Randomized Controlled Trials. Wound Manag Prev 65(2): 30–8
10. Cui CY, Schlessinger D (2015) Eccrine sweat gland development and sweat secretion. Exp Dermatol 24(9): 644–50
11. Dang W, Liu Y, Zhou Q et al (2021) Risk factors of medical device-related pressure injury in intensive care units. J Clin Nurs 10.1111/jocn.15974
12. Dąbrowska AK, Rotaru GM, Derler S et al (2019) Materials used to simulate physical properties of human skin. Skin Res Technol 22(1): 3–14
13. Dai A, Wang S, Zhou L et al (2019) In vivo mechanical characterization of human facial skin combining curved surface imaging and indentation techniques. Skin Res Technol 25(2): 142–9
14. European Pressure Ulcer Advisory Panel, National Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance (2019) Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline 2019. Available at: https://bit. ly/3rOvsW0 (accessed 15.02.2022)
15. Ferrari F, Bertoni M, Caramella C, Waring MJ (1994) Comparative evaluation of hydrocolloid dressings by means of water uptake and swelling force measurements: I. Int J Pharm 112(1): 29–36
16. Ferrari F, Bertoni M, Bonferoni MC et al (1995) Comparative evaluation of hydrocolloid dressings by means of water uptake and swelling force measurements: II. Int J Pharm 117(1): 49–55
17. Flynn C, Taberner AJ, Nielsen PMF, Fels S (2013) Simulating the three-dimensional deformation of in vivo facial skin. J Mech Behav Biomed Mater 28: 484–94
18. Gefen A, Alves P, Creehan S et al (2019) Computer modeling of prophylactic dressings: An indispensable guide for healthcare professionals. Adv Skin Wound Care 32(7S Suppl 1): S4–S13
19. Gefen A, Alves P, Ciprandi G et al (2020) Device-related pressure ulcers: SECURE prevention. J Wound Care 29(Sup2a): S1–S52
20. Gefen A (2021) The aetiology of medical device-related pressure ulcers and how to prevent them. Br J Nurs
21. Gefen A, Brienza DM, Cuddigan J et al (2021) Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int Wound J doi: 10.1111/iwj.13667 [Online ahead of print] 30(15): S24–S30
22. Hao DF, Feng G, Chu WL et al (2015) Evaluation of effectiveness of hydrocolloid dressing vs ceramide containing dressing against pressure ulcers. Eur Rev Med Pharmacol Sci 19(6): 936–41
23. Hasatsri S, Pitiratanaworanat A, Swangwit S et al (2018) Comparison of the morphological and physical properties of different absorbent wound dressings. Dermatol Res Pract 2018: 9367034
24. Jackson D, Sarki AM, Betteridge R, Brooke J (2019) Medical device-related pressure ulcers: A systematic review and meta-analysis. Int J Nurs Stud 92: 109–120
25. Jessen C (2000) Temperature Regulation in Humans and Other Mammals (1st edn.) Berlin: Springer-Verlag Berlin Heidelberg pp45
26. Jin SG, Yousaf AM, Kim KS et al (2016) Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressing. Int J Pharm 501(1-2): 160–6
27. Kalra A, Lowe A, Al-Jumaily AM (2016a) Mechanical behaviour of skin: A review. J Material Sci Eng 5(4): 1–7
28. Kalra A, Lowe A, Al-Jumaily AM (2016b) An overview of factors affecting the skin’s Young’s modulus. J Aging Sci 4(2): 1–5
29. Lanel B, Barthès-Biesel D, Regnier C, Chauvé T (1997) Swelling of hydrocolloid dressings. Biorheology 34(2): 139–53
30. Levy A, Schwartz D, Gefen A (2017) The contribution of a directional preference of stiffness to the efficacy of prophylactic sacral dressings in protecting healthy and diabetic tissues from pressure injury: computational modelling studies. Int Wound J 14(6): 1370–7
31. Lim SA, Lee DE (2003) Comparison of usual applicating foam dressing materials in split thickness skin graft donor site. Journal of Korean Burn Society 6(1): 45–51
32. Lin YJ , Lee GH , Chou CW et al (2015) Stimulation of wound healing by PU/hydrogel composites containing fibroblast growth factor-2. J Mater Chem B 3(9): 1931–41
33. Luboz V, Promayon E, Payan Y (2014) Linear elastic properties of the facial soft tissues using an aspiration device: towards patient specific characterization. Ann Biomed Eng 42(11): 2369–78
34. Lustig A, Margi R, Orlov A et al (2021) The mechanobiology theory of the development of medical device-related pressure ulcers revealed through a cell-scale computational modeling framework. Biomech Model Mechanobiol 20(3):851-860
35. McKee CT, Last JA, Russell P, Murphy CJ (2011) Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng Part B Rev 17(3): 155–64
36. Moore ZE, Webster J (2018) Dressings and topical agents for preventing pressure ulcers. Cochrane Database Syst Rev (8):CD009362
37. Montain SJ, Cheuvront SN, Lukaski HC (2007) Sweat mineral-element responses during 7 h of exercise heat stress. Int J Sport Nutr Exerc Metab 17(6): 574–82
38. Omura Y, Yamabe M, Anazawa S (2010) Peristomal skin disorders in patients with intestinal and urinary ostomies: influence of adhesive forces of various hydrocolloid wafer skin barriers. J Wound Ostomy Continence Nurs 37(3): 289–98
39. Otero DP, Domínguez DV, Fernández LH et al (2017) Preventing facial pressure ulcers in patients under non-invasive mechanical ventilation: a randomised control trial. J Wound Care 26(3): 128–36
40. Orlov A, Gefen A (2021) How influential is the stiffness of the foam dressing on soft tissue loads in negative pressure wound therapy? Med Eng Phys 89: 33–41
41. Peko Cohen L, Ovadia-Blechman Z, Hoffer O, Gefen A (2019) Dressings cut to shape alleviate facial tissue loads while using an oxygen mask. Int Wound J 16(3): 813–26
42. Pailler-Mattei C, Bec S, Zahouani H (2008) In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys 30(5): 599–606
43. Sanusi AL (2011) Severe wound traction-blisters after inadequate dressing application following laparoscopic cholecystectomy: case report of a preventable complication. Patient Saf Surg 5(1): 4
44. Schwartz D, Gefen A (2019) The biomechanical protective effects of a treatment dressing on the soft tissues surrounding a non-offloaded sacral pressure ulcer. Int Wound J 16(3): 684–95
45. Sieracki J, Wilkes R, Bennett ER, McNulty AK (2020) Finite element analysis modeling of a novel silicone dressing. Cureus 12(9): e10629
45. Walker RM, Ayello EA, Chan SC et al (2019) Device related pressure injuries. In: European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. EPUAP/NPIAP/PPPIA, Westford, MA, USA. pp181–93. Available at: https://bit.ly/3gOgFo0 (accessed 15.02.2022)
46. Yamaguti WP, Moderno EV, Yamashita SY et al (2014) Treatment-related risk factors for development of skin breakdown in subjects with acute respiratory failure undergoing noninvasive ventilation or CPAP. Respir Care 59(10): 1530–6
47. Yamane T, Nakagami G, Yoshino S et al (2015) Hydrocellular foam dressings promote wound healing associated with decrease in inflammation in rat periwound skin and granulation tissue, compared with hydrocolloid dressings. Biosci Biotechnol Biochem 79(2): 185–9
48. Zahouani H, Pailler-Mattei C, Sohm B et al (2009) Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Skin Res Technol 15(1): 68–76
49. Zeitz K, McCutcheon H (2005) Tradition, rituals, and standards, in a realm of evidenced based nursing care. Contemp Nurse 18(3): 300–8
50. Zheng YP, Mak A (1999) Effective elastic properties for lower limb soft tissues from manual indentation experiment. IEEE Trans Rehabil Eng 7(3): 257–67
This article is excerpted from the Wounds International 2022 | Vol 13 Issue 1 by Wound World.



