Preamble
The following updated statement is issued by the American Society for Metabolic and Bariatric Surgery (ASMBS) in response to numerous inquiries made to the Society by patients, physicians, society members, hospitals, and others regarding single-anastomosis duodenal switch as a treatment for obesity and metabolic disease. This recommendation is based on current clinical knowledge, expert opinion, and published peer-reviewed scientifific evidence available at this time. The statement is not intended as, and should not be construed as, stating or establishing a local, regional, or national standard of care.
Endorsement process
The ASMBS has a standard pathway and process for the endorsement (or removal of endorsement) of procedures. That pathway, summarized here, was used in the endorsement of single-anastomosis duodenoileal bypass with sleeve gastrectomy. Further details may be found on the society website [1].
1. Application by an ASMBS member sponsor in active practice for a new procedure or removal of an endorsed procedure. Multiple ASMBS member co-sponsors are allowed and encouraged.
2. Primary Executive Committee of the Executive Council review: 75% endorsement required for next stage. This review will be inclusive and mainly to ensure plausibility of new procedure and device before invoking full review.
3. Application assessed by the ASMBS Pathway for Endorsement of New Devices and Procedures Committee. The Pathway for Endorsement of New Devices and Procedures Committee will include the chairs of clinicalissues, insurance, quality improvement, and patient safety, emerging technology and integrated health president or their designee. In the course of their review, a clinical issues position statement may be produced concurrently.
4. Application presented to Executive Council by ASMBS member sponsor and 1 co-sponsor and pro and con advocates from Pathway for Endorsement of New Devices and Procedures Committee.
5. Executive council review and open vote: 75% endorsement required to next stage.
6. ASMBS member comment of new procedure/device application with Pathway for Endorsement of New Devices and Procedures Committee summary.
7. Final EC vote: 75% endorsement required for final affirmation.
8. Outcome of endorsement sent to major insurers and Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program once application endorsed.
The single-anastomosis duodenal switch, also known as the loop duodenal switch, stomach intestinal pylorussparing surgery, and most descriptively single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S),was first reviewed by the American Society for Metabolic and Bariatric Surgery (ASMBS) as a new metabolic and bariatric procedure in a published position statement in 2016 [2]. The intention of the SADI-S procedure was to address certain limitations and complexities inherent to other standard bariatric and metabolic procedures, including inadequate weight loss, weight regain, variable improvement of weight-related co-morbidities, hypoabsorptive complications, internal hernias, and technical difficulty. The biliopancreatic diversion with duodenal switch (BPD-DS), a pylorus-sparing modification of Scopinaro’s original BPD, was reported as an open procedure by Hess and Hess, first in 1998 [3,4]. The BPD-DS was intended to decrease the rates of marginal ulceration and dumping seen with the BPD, as well as issues with severe hypoabsorption and diarrhea. In the Hess’ description of their procedure, the length of the entire small intestine is measured, after which the alimentary limb length is 40% of the total and the common channel length is 10% of the total. The sleeve gastrectomy (SG) was performed over a 40-Fr dilator allowing 1 to 1.5 fingerbreadths additional width and then stapled, with the staple line being inverted with Lembert sutures. The intention was to ultimately have an SG volume of 100 mL. The BPD-DS, now generally performed laparoscopically, is associated with the greatest weight loss and remission of diabetes of the currently performed conventional metabolic and bariatric procedures that are endorsed by the ASMBS.
Technical complexity and the risk of long-term nutritional deficiencies have limited the acceptance and popularity of BPD-DS. The most recent published estimate of bariatric surgery procedures from 2018 showed the BPDDS accounted at the time for only .8% of all such procedures in the United States [5]. The SADI-S technique was first described in 2007 as a simplification of the BPD-DS, beginning with the creation of an SG but replacing the Roux-en-Y reconstruction with a single-anastomosis duodenoileostomy with a longer 200-cm common or “absorptive” channel [6]. This channel length was later increased to 250 cm because of an unacceptably high rate of hypoalbuminemia and other hypoabsorptive complications [7]. Modifications of SADI-S have also erratically decreased the bougie size used to create the SG to improve weight loss, but with a concurrent increase in common length to 300 cm to minimize hypoabsorption [8–10]. Of note, to avoid confusion, this statement will not cover a variety of even less commonly performed procedures, which involve a single anastomosis between the duodenum and the jejunum. Short- and medium-term data on SADI-S At the time of our original statement, there were only 4 published studies on SADI-S, comprising a total of 222 patients, with follow-up for individual patients ranging from 18 months to 5 years [6–9]. Three of these studies represented a single institution’s ongoing consecutive series, while the fourth came from a center that compared SADI-S with a matched Roux-en-Y gastric bypass (RYGB) cohort. Both sites involved surgeons with prior extensive BPD-DS experience.
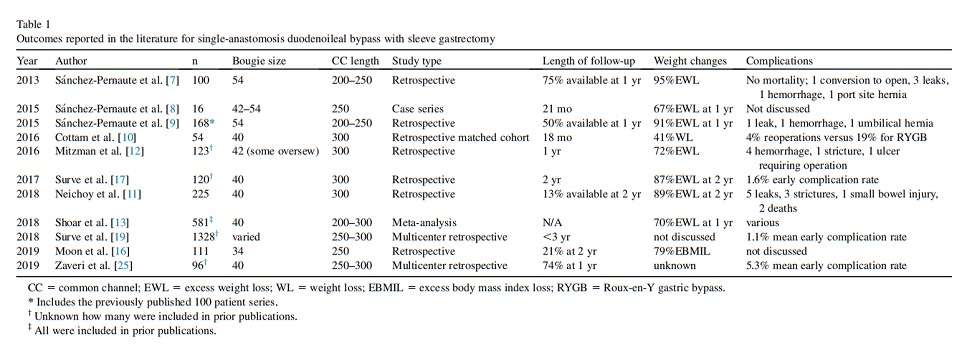
Since that time, Neichoy et al. [11] expanded on their center’s retrospective results. Among 225 patients undergoing SADI-S with a 40-Fr SG and a 300-cm absorptive limb, 30 patients were available for follow-up at 24 months; among these patients, the percent excess body mass index loss was 88.8 6 20.2%. There were 6 deaths among the cohort (2.7%) but the authors felt only 3 deaths were attributable to the surgery and quoted a mortality rate of 1.3%, with 2 deaths occurring within 30 days of surgery. The same group, publishing as Mitzman et al. [12], reported retrospectively on a different group as follows: 123 SADIS patients with a 42-Fr sleeve and a 300-cm common channel, with an average follow-up of 1 year. Mean percent excess weight loss (%EWL) at that time was 72%. Six patients required early reoperation, but no deaths reported [12].
One systematic review of the literature on SADI-S published in 2018 produced 12 eligible studies comprising a total of 581 patients. These reports [6–9], included primary and revisional SADI-S, with a variety of absorptive limb lengths (not always reported), bougie sizes, and anastomotic techniques. The longest reported follow-up of individual patients was 5 years, although the total number who reached this point is unknown [13].
Comparison of SADI-S with other bariatric procedures An early comparison of 54 SADI-S patients and 54 RYGB patients showed the groups had statistically similar weight loss at 18 months (39.6 versus 41% total weight loss, respectively), which the authors did not choose to explain. For clarification, the RYGBs were performed with a 25-mm end-to-end anastomosis stapler for the gastrojejunostomy and a 150-cm Roux limb; the SADI-S group had a 40-Fr SG and a 300-cm common channel. There was more nausea, marginal ulceration, and need for subsequent endoscopies among the RYGB patients [10].
The same center’s analysis of a sex- and body mass index–matched cohort of 53 SG patients and 53 SADI-S patients with 300-cm absorptive limbs (all with a 40-Fr SG) showed early weight loss seems to be related to the SG component, while the intestinal component extended the period of ongoing weight loss by several months; thus, increasing the total %EWL significantly. Complication rates were noted to be similar between the 2 groups, with most complications being related to the SG component of each procedure [14].
This center’s matched-cohort analysis comparing 61 SADI-S patients (40-Fr SG/300-cm absorptive limb) with 61 BPD-DS patients (40-Fr SG/150-cm Roux limb/150- cm common channel) at 2-year follow-up showed %EWL and rate of complications was statistically identical between the 2 procedures, but the mean operative time for SADI-S was significantly shorter (70 6 14 versus 137 6 36 min) [15]. Similarly, Moon et al. [16] compared 111 SADI-S patients with 74 BPD-DS patients in a 2015 to 2017 review. The weight loss and the complication rates were very similar between groups.
Another retrospective analysis, containing patients who had already been analyzed in prior studies, compared 62 BPD-DS patients (performed 2011–2013; 46-Fr SG, 150 cm/150 cm) with 122 SADI-S patients (performed 2013– 2015; 40-Fr SG/300 cm). A total of 99 patients were available for 2-year follow-up, at which time %EWL was significantly greater for the BPD-DS than for the SADI-S patients. There were no deaths among the groups but there were significantly more short- and long-term complications among the patients undergoing BPD-DS, and a significantly longer length of stay for BPD-DS compared with SADI-S (4.1 6 6.2 versus 2 6 1 d). Limitations of this study include the fact that the surgeons had substantially more experience at the time of performing SADI-S, and that SG size was different between the groups [17].
Torres et al. [18] reported on 10 years’ experience comparing SADI-S with BPD-DS and RYGB. Of 106 SADI-S patients seen at 3 years, the mean percentage of total weight loss (%TWL) was 38.7 6 10.7, and among 149 RYGB patients seen at the same time point, %TWL was 28.7 6 9.7, a statistically significant difference. Among their patients with type 2 diabetes, 97 RYGB patients lost 30.3 6 7.1%TWL; 97 SADI-S patients lost 35.5 6 6.7 %TWL; and 77 BPDDS patients lost 35.2 6 10.5 %TWL. Improvement in measures of type 2 diabetes were statistically the same between the SADI-S and BPD-DS groups, both of which performed statistically better in this realm than did RYGB. The advantages of SADI-S in the opinion of the authors included its being easier to perform, easier to dismantle, and having a lower rate of internal herniation than BPD-DS [18].
The largest retrospective, multicenter review of SADIS complications studied 1328 patients with 1- to 6-year follow-up from 9 expert centers in different countries [19]. Of note, many of the patients in the database had been included in prior publications, and the data set again included patients with a variety of absorptive limb lengths, SG bougie sizes, and anastomotic techniques. The authors’ conclusions were that the rates of anastomotic complications (leak, obstruction, ulcer, stricture, bile reflux) were lower among their SADI-S patients than comparable rates reported in the literature for both RYGB and BPD-DS. There was no way to determine statistical significance by the nature of this study. The authors did note that there was evidence of partial small bowel obstruction in 2 patients in the series because of retrograde filling of the afferent limb [20]. Tacking the afferent limb to the gastric antrum reportedly eliminated this complication in the series. The authors noted that there were no reported cases of internal hernia or volvulus after primary SADI-S, which they proposed to be related to the presence of one less anastomosis; there has been only a single case report of internal hernia in a revisional SADI-S [21].
Even with a 300-cm absorptive limb, some patients have been reported to be troubled with chronic diarrhea, defined by Surve et al. [22] as at least 4 loose stools per day for at least 4 weeks, with no improvement after dietary changes, probiotics, and medications. This group opted to increase 2 patients’ absorptive limb length to 500 cm [22], which resulted in the creation of a duodenojejunostomy. Both patients had significant improvement in diarrhea immediately after surgery. What is not mentioned is the likely technical difficulty of transecting a duodenoileostomy and creating a new duodenojejunostomy; for surgeons hoping to adopt SADI-S on account of its relative technical ease, the need for future SADI-S revision could create a significant challenge.
Also not addressed among any of the SADI-S studies is the percent of total small bowel length the common channel represents. There is no mention in any paper of counting out the complete small bowel length, which is known to vary greatly depending on sex, age, height, and weight. One study of 91 laparotomy patients, in which the small intestine was measured from ligament of Treitz to ileocecal valve using a 10-cm ruler on the antimesenteric border with no stretch, found a mean small bowel length of 998 cm, but with great variability, between 630 and 1510 cm [23]. Clearly, a 300-cm common channel in 1 patient could represent a significant amount of total bowel length, but in another could be a much smaller percentage. This discrepancy makes comparison between patients and procedures difficult. Measuring bowel length during a complex laparoscopic operation, unlike in an open procedure, can be tedious and potentially harmful. One potential solution is to perform preoperative magnetic resonance enterography, which has been shown to accurately calculate small bowel length [24].
The pertinent studies on SADI-S outcomes are shown in Table 1.
SADI-S as a reoperative procedure
SADI-S has been used as a conversional procedure for inadequate weight loss after RYGB. One group reported a retrospective study of 32 RYGB patients who failed to maintain .50% EWL after an average of 16.4 6 9.3 years [26]. Among the 32 patients, 9 underwent BPD-DS (40-Fr SG/ 150-cm alimentary limb/150-cm common channel) and 23 underwent SADI-S (40-Fr SG/300-cm absorptive limb). Only 11 patients had data collected at 2 years after surgery; %EWL was neither statistically different between the 2 groups (67.5% versus 54.5%, respectively), nor were their complication rates statistically different. It is important to note the vast majority of bariatric surgeons who perform RYGB do not perform conversions to BPD-DS, so even among experts the reported numbers are small.
SADI-S has also been advocated for patients with weight regain or insufficient weight loss after SG and there is a growing body of literature on the need for SG revisions and conversions [10,27]. Some advocate SADI-S after suboptimal outcome from SG as producing better and more reliable weight loss than other procedures, such as RYGB [25,28–31].
Summary and recommendations
The SADI-S procedure is fundamentally a variant of the DS operation, in which the transected duodenum is anastomosed to a loop of ileum as opposed to the classic DS in which a Roux-en-Y confifiguration is used. The SADI-S procedure was developed in part to reduce the complexity and therefore the risks of performing a Roux-en-Y configuration with small diameter distal bowel and a need for 2 anastomoses. In addition, many published reviews of the procedure have advocated a longer length of distal common channel than typically recommended in the classic DS procedure. Most recent recommendations suggest a common channel length of no less than 300 cm, but SG sizes vary widely, from 34 to 54 Fr [32]. The primary difference between the classic DS and the loop configuration for DS involves the issue of so-called “biliopancreatic diversion.” As such, the classic DS diverts the flow of biliopancreatic secretions distally by virtue of the Roux-en-Y configuration, whereas the loop DS configuration does not. It is not known whether or not this difference provides for different risks and/or benefits, in particular the question of bile reflux.
The ASMBS endorses the classic DS procedure (BPDDS) and it is listed among the procedures that the society believes meet appropriate standards for safety and benefit. The SADI-S procedure, as a variant of classic DS therefore merits consideration for ASMBS endorsement as a modi-fication of an already-endorsed metabolic/bariatric procedure. As such, it is reasonable to consider the SADI-S could be considered for endorsement with less available published peer-reviewed data than would be required for an entirely novel surgical procedure for which no predicate procedure exists.
With additional publications reporting outcomes of many more patients who have undergone SADI-S since the previous ASMBS statement (amounting to a total of w1500 currently reported patients), the ASMBS has reached the conclusion that SADI-S provides for similar outcomes to those reported after classic DS and should therefore be endorsed, similar to the ASMBS’ endorsement of the predicate procedure of BPD-DS. The conclusion from the current review is that the currently available peer-reviewed literature does not suggest outcomes will differ substantially from those seen with classic DS.
The ASMBS will continue to monitor and evaluate emerging data on this procedure and, when appropriate, will issue an updated evidence-based position statement at a future time. The following recommendations are currently endorsed by the ASMBS regarding SADI-S for the primary treatment of obesity or metabolic disease:
1. SADI-S, a modification of classic Roux-en-Y DS, is therefore endorsed by ASMBS as an appropriate metabolic bariatric surgical procedure.
2. Publication of long-term safety and efficacy outcomes is still needed and is strongly encouraged, particularly with published details on SG size and common channel length.
3. Data for these procedures from accredited centers should be reported to the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database and separately recorded as single-anastomosis DS procedures to allow for accurate data collection.
4. There remain concerns about intestinal adaptation, nutritional issues, optimal limb lengths, and long-term weight loss/regain after this procedure. As such, ASMBS recommends a cautious approach to the adoption of this procedure, with attention to ASMBS-published guidelines on nutritional and metabolic support of bariatric patients, in particular for DS patients [33,34].
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
[1] Pathway for endorsement for new devices and procedures [monograph on the Internet]. Newberry: American Society for Metabolic and Bariatric Surgery; c2020 [cited 2020 Mar 16]. Available from: https://asmbs.org/pathway-for-endorsement-for-new-devices-and-procedures.
[2] Kim J, for the American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. American Society for Metabolic and Bariatric Surgery statement on single-anastomosis duodenal switch. Surg Obes Relat Dis 2016;12(5):944–5.
[3] Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg 1998;8(3):267–82.
[4] Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg 2005;15(3):408–16.
[5] English WJ, DeMaria EJ, Hutter MM, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis 2020;16(4):457–63.
[6] Sanchez-Pernaute A, Rubio Herrera MA, Perez-Aguirre E, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg 2007;17(12):1614–8.
[7] Sanchez-Pernaute A, Rubio MA, Perez-Aguirre E, Barabash A, Cabrerizo L, Torres A. Single-anastomosis duodenoileal bypass with sleeve gastrectomy: metabolic improvement and weight loss in first 100 patients. Surg Obes Relat Dis 2013;9(5):731–5.
[8] Sanchez-Pernaute A, Rubio MA, Conde M, Arrue E, PerezAguirre E, Torres A. Single-anastomosis duodenoileal bypass as a second step after sleeve gastrectomy. Surg Obes Relat Dis 2015;11(2):351–5.
[9] Sanchez-Pernaute A, Rubio MA, Cabrerizo L, Ramos-Levi A, PerezAguirre E, Torres A. Single-anastomosis duodenoileal bypass with sleeve gastrectomy (SADI-S) for obese diabetic patients. Surg Obes Relat Dis 2015;11(5):1092–8.
[10] Cottam A, Cottam D, Medlin W, et al. A matched cohort analysis of single anastomosis loop duodenal switch versus Roux-en-Y gastric bypass with 18-month follow-up. Surg Endosc 2016;30(9):3958–64.
[11] Neichoy BT, Schniederjan B, Cottam DR, et al. Stomach intestinal pylorus-sparing surgery for morbid obesity. JSLS 2018;22(1):e2017.00063.
[12] Mitzman B, Cottam D, Goriparthi R, et al. Stomach intestinal pylorus sparing (SIPS) surgery for morbid obesity: retrospective analyses of our preliminary experience. Obes Surg 2016;26(9):2098–104.
[13] Shoar S, Poliakin L, Rubenstein R, Saber AA. Single anastomosis duodeno-ileal switch (SADIS): a systematic review of efficacy and safety. Obes Surg 2018;28(1):104–13.
[14] Cottam A, Cottam D, Roslin M, et al. A matched cohort analysis of sleeve gastrectomy with and without 300 cm loop duodenal switch with 18-month follow-up. Obes Surg 2016;26(10):2363–9.
[15] Cottam A, Cottam D, Portenier D, et al. A matched cohort analysis of stomach intestinal pylorus saving (SIPS) surgery versus biliopancreatic diversion with duodenal switch with two-year follow-up. Obes Surg 2017;27(2):454–61.
[16] Moon RC, Kirkpatrick V, Gaskins L, Teixeira AF, Jawad MA. Safety and effectiveness of single- versus double-anastomosis duodenal switch at a single institution. Surg Obes Relat Dis 2019;15(2):245–52.
[17] Surve A, Zaveri H, Cottam D, Belnap L, Cottam A, Cottam S. A retrospective comparison of biliopancreatic diversion with duodenal switch with single anastomosis duodenal switch (SIPS-stomach intestinal pylorus sparing surgery) at a single institution with two year follow-up. Surg Obes Relat Dis 2017;13(3):415–22.
[18] Torres A, Rubio MA, Ramos-Levı AM, Sanchez-Pernaute A. Cardiovascular risk factors after single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S): a new effective therapeutic approach? Curr Atheroscler Rep 2017;19(12):1–8.
[19] Surve A, Cottam D, Sanchez-Pernaute A, et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis 2018;14(5):594–602.
[20] Surve A, Zaveri H, Cottam D. Retrograde filling of the afferent limb as a cause of chronic nausea after single anastomosis loop duodenal switch. Surg Obes Relat Dis 2016;12(4):e39–42.
[21] Summerhays C, Cottam D, Cottam A. Internal hernia after revisional loop duodenal switch surgery. Surg Obes Relat Dis 2015;12(1):e13–5.
[22] Surve A, Zaveri H, Cottam D. A step-by-step surgical technique video with two reported cases of common channel lengthening in patients with previous stomach intestinal pylorus sparing surgery to treat chronic diarrhea. Surg Obes Relat Dis 2017;13(4):706–9.
[23] Raines D, Arbour A, Thompson HW, Figueroa-Bodine J, Joseph S. Variation in small bowel length: factor in achieving total enteroscopy? Dig Endosc 2015;27(1):67–72.
[24] Sinha R, Trivedi D, Murphy PD, Fallis S. Small-intestinal length measurement on MR enterography: comparison with in vivo surgical measurement. Am J Roentgenol 2014;203(3):274–9.
[25] Zaveri H, Surve A, Cottam D, et al. A multi-institutional study on the mid-term outcomes of single anastomosis duodeno-ileal bypass as a surgical revision option after sleeve gastrectomy. Obes Surg 2019;29(10):3165–73.
[26] Surve A, Zaveri H, Cottam D, Belnap L, Medlin W, Cottam A. Midterm outcomes of gastric bypass weight loss failure to duodenal switch. Surg Obes Relat Dis 2016;12(9):1663–70.
[27] Felsenreich DM, Langer FB, Kefurt R, et al. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2016;12(9):1655–62.
[28] Lee Y, Ellenbogen Y, Doumouras AG, Gmora S, Anvari M, Hong D.Single- or double-anastomosis duodenal switch versus Roux-en-Y gastric bypass as a revisional procedure for sleeve gastrectomy: a systematic review and meta-analysis. Surg Obes Relat Dis 2019;15(4):556–66.
[29] Moon RC, Fuentes AS, Teixeira AF, Jawad MA. Conversions after sleeve gastrectomy for weight regain: to single and double anastomosis duodenal switch and gastric bypass at a single institution. Obes Surg 2019;29(1):48–53.
[30] Dijkhorst PJ, Boerboom AB, Janssen IMC, et al. Failed sleeve gastrectomy: single anastomosis duodenoileal bypass or Roux-en-Y gastric bypass? A multicenter cohort study. Obes Surg 2018;28(12):3834–42.
[31] Balibrea JM, Vilallonga R, Hidalgo M, et al. Mid-term results and responsiveness predictors after two-step single-anastomosis duodenoileal bypass with sleeve gastrectomy. Obes Surg 2017;27(5):1302–8.
[32] Topart P, Becouarn G. The single anastomosis duodenal switch modifications: a review of the current literature on outcomes. Surg Obes Relat Dis 2017;13(8):1306–12.
[33] Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient - 2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013;9(2):159–91.
[34] Parrott J, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health nutritional guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis 2017;13(5):727–41.
This article is excerpted from the American Society for Bariatric Surgery by Wound World.


