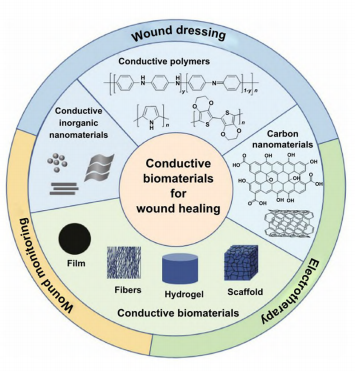
1 Introduction
As the largest organ of the human body, skin displays significant influence on diverse human activities and functions, including protection from pathogens, sensing of external environment, and thermoregulation [1]. However, laying on the outmost of the human body and facing incessant conflicts, skin owing to its elastic and soft nature is susceptible to generate defects that are referred to as wounds [2, 3]. Even though human skin could self-repair spontaneously to restore its structural and functional integrity, wound care is still of great necessity to prevent infection and desiccation, alleviate pain, protect the open site, accelerate the healing process, and avoid scar formation, especially for large and open wounds or burns [4 -6]. In 2014, 17.2 million hospital visits by acute wounds were recorded in the USA [7]. Currently, about 1- -2% of the population in developed countries suffers from chronic wounds [8]. Meanwhile, ascribing to diseases, aging or inappropriate treatment, chronic wounds as diabetic ulcers, vascular ulcers, and pressure ulcers possessing a long and cruel healing period do not only affect patients' daily life but also associate with high morbidity and mortality [9, 10]. The global advanced wound care products market is growing rapidly, about $12 billion in 2020 and estimated to be $18.7 billion by 2027 [7]. At present, wound healing remains a hot and challenging issue both in clinic and scientific research.
To date, the physiology of wound healing has been well established [11]. The healing process comprises four overlapping phases: hemostasis, inflammation, proliferation, and remodeling, along with diverse growth factors, enzymes, and cytokines exerting significant effects in synergistically modulating relevant cell activities [5, 12-14]. There are many types of wounds: acute incision and excision wound can go through normal healing process, while chronic wounds have aberrant healing conditions [10]. In clinic, wound healing management varies according to the tissue feature, the intrinsic regenerative capacity, wound classification, and other environmental variables [6, 10, 15-17]. The therapeutic strategies for wounds are versatile, including hyperbaric oxygen therapy [18- 20], negative- pressure therapy [21], vacuum-assisted closure [22], ultrasound [23], electrotherapy [24, 25], auto/allograft and xenograft [4, 26,27], cell-based therapy and engineered skin graft [4, 28, 29], and topical drug and growth factor delivery [30- -32]. No
matter which class the wounds belong to, and which wound care strategy would be chosen, wound dressing is requisite [33]. Traditional passive wound dressings like gauze, bandage, and cotton wool could hardly fit the open wounds and exert no active effect in wound healing [9]. Even worse, they would adhere to the skin tissue, causing dehydration and second injury upon replacement. In contrast, modern biomaterial-based wound dressings integrating multifunction as maintaining a moist environment, managing exudate and protection from pathogens, antibacterial capacity, antioxidant property, injectability, self-healing capacity, adhesiveness, and suitable mechanical properties have recently surged and demonstrated extraordinary advantages in more complicated situations [4, 34. 35]. Except for the above-mentioned features, our human skin possesses another key characteristic, the conductive nature of the intact skin, which plays a vital role in human activities [36- -38]. Once the skin is disrupted, endogenous wound-induced electric fields ranging from 40- 200 mV/mm generate and immediately initiate wound healing [39 42]. Accordingly, electrical stimulation-based electrotherapy has been developed and applied in practice since the end of the
twentieth century, especially for chronic wounds. The electrical stimulation (ES) accelerates the wound healing process in all stages through diverse pathways [39- 41, 43- 45]. It can alleviate edema around the electrode, guide keratinocyte migration, enhance re- epithelialization, direct dermal angiogenesis, modulate a variety of genes relevant with wound healing, and generate antibacterial effects [43, 46, 47]. But the application of electrotherapy must be carried out withalarge extracorporeal ES device and requires careful and precise evaluation of the relevant parameters, including voltage, current, mode, and working time, depending on the nature and condition of the wound [48, 49]. Moreover, the efficacy of electrotherapy is limited by the uneven distribution of ES ascribing to a large amount of body fluid, irregular wound shape, the exudates induced metal electrodes corrosion, and wound status [24, 25, 50].
In the past decade, wound treatment strategy relating to the conductive nature of human skin has emerged and attracted much attention for its high efficacy, processing flexibility, and ease in handling and management. The sophisticated conductive biomaterial-based wound dressings with similar conductivity to that of human skin have demonstrated significant enhancement in wound healing and exhibited great potential in different types of wounds, as full-thickness acute wound, infected wound, and diabetic wound [37, 51, 52]. The basic principle of fabricating conductive wound dressing is to incorporate the electroactive
substances mainly including carbon nanomaterial [5, 53, 54], conductive polymers (CPs) [55, 56], or metal-based materials [57- -60] into the polymeric biomaterial [51]. So far, there have designed a lot of conductive wound dressings in different forms, as film, membrane, electrospun nanofiber, hydrogel, cryogel, and foam [37, 61, 62]. Besides, since the advanced development of tissue engineering and regenerative medicine, conductive scaffolds mimicking ECM loaded with bioactive molecules or cells have also been delicately developed for more severe wounds as large or open wounds and chronic wounds with poor regenerative abilities, providing mechanical support and modulating cell activities [63,64]. Usually, sponge and foam, hydrogel, and nanofibrous network with highly porous structures are ideal for scaffolds
[65]. However, a comprehensive review summarizing the design and application of conducive biomaterials for wound healing and skin tissue engineering is still lacking.
This review article comprehensively summarizes the recent immense achievement and great potential of the conductive wound dressings for wound healing and skin tissue engineering. The advantages and drawbacks of different conductive substances are summarized (Fig. 1). The current
fabrication methods of conductive biomaterial in different forms are reviewed and classified by 2D and 3D morphologies (Fig. 1), as well as their applications in different wound models and the related aspects to consider when facing specific wound were deliberated. Three main strategies of conductive biomaterials in promoting wound healing were summarized. The mechanism of conductive materials on accelerating wound healing is also discussed. Commonly, conductive materials do not work alone, but are synergistically utilized with other functional materials, thereby the combination are also recorded, and the synergistic effects are discussed (Fig.1). Furthermore, considering the current achievements and clinical needs, we envision the future directions and propose the challenges of conductive biomaterials in wound healing and skin tissue engineering.
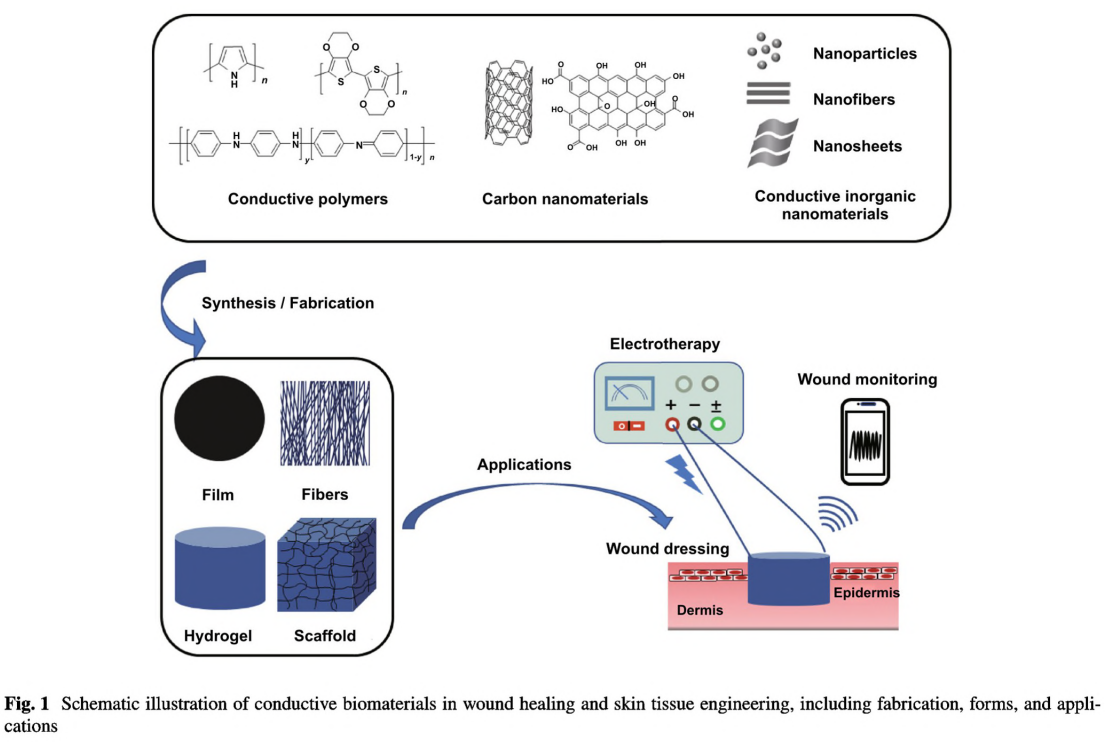
2 General Design Principles of Conductive Biomaterials for Wound Healing
Three main issues need to be carefully evaluated when designing conductive biomaterials for wound healing. First is the selection of matrix material. Naturally derived polymers have good biocompatibility, enzymatic or hydrolytic reactions-based biodegradability, and versatile biological properties which could promote wound healing, but their quality varies from batch to batch [56, 66]. In contrast, synthetic polymers have more controlled structure and superior mechanical properties, but poor cell attachment owing to their hydrophilicity [67]. Biocompatibility and degradability are other two critical issues that impair the biomedical applications of synthetic polymers [68, 69]. Besides, they usually lack bioactivity and need to be functionalized to meet biological requirements. Therefore, in numerous cases, natural and synthetic polymers are combined either through simple blending or crosslinking to integrate multifunction, including biocompatibility, degradability, and specific bioactivity and suitable mechanical strength. So far, various natural and synthetic polymeric materials have been utilized to fabricate wound dressings, including chitosan, fibrin, gelatin, hyaluronic acid, alginate, cellulose, silk fibroin, polyethylene glycol, polycaprolactone, poly(actic acid), poly(glycolic acid), polyvinyl alcohol, and polyurethane [37, 70, 71]. These biomaterials exhibit diverse properties due to the intrinsic unique features of the components and the fabrication methods.
Except for the composition, the structural morphology is another key factor for biomaterials that notably determines the fabrication method, properties, and performance [9]. Commonly, film, membrane, and fibers possessing 2D structure have good oxygen permeability, resistance toward water and tough mechanical properties. Sponges, foam, and hydrogel owning 3D network and porous structure could
absorb large amount of exudate, maintain moist environment, and act as carriers for bioactive substances and cells [72, 73]. The applications of these biomaterials also depend on their morphology. 2D biomaterials are usually used as wound dressing, while those with 3D structure can be fabricated as wound dressings and tissue scaffolds. Particularly, as nanofibers could be programmed into ECM-liked aligned
architecture, they also have great potential in skin scaffolds [26]. Meanwhile, the application of these conductive biomaterials is somewhat limited by their morphology. 2D biomaterials cannot adapt to deep and chronic wounds, and foams with tough nature could not be applied on delicate skin and dry wound, while hydrogel with relative soft nature may cause wound dehydration and fail long-term use due to inevitable water evaporation and the constant movement of the wounds [9]. It is worth noting that in the cases of the combination of natural and synthetic polymers, the structural morphology of these biomaterials also affects the selection of fabrication methods. Specifically simple blending could be found in fabricating 2D biomaterials [74]. Chemical or physical crosslinking is an alternative utilized to further enhance the stability and mechanical properties. Meanwhile, crosslinking is essential when designing 3D biomaterials, where permanent covalent bonds usually result in a tough matrix, while physical crosslinking and dynamic covalent
bonds usually result in soft biomaterials.
Third comes to the incorporation of conductive materials. So far, several types of conductive materials have been elaborately studied and explored. They can be well-tuned and controlled into different morphologies, as nanoparticles, nanowires, nanotubes, and nanosheets, which has a significant impact on their properties [53, 75, 76]. The excellent electroconductivity, unique optical properties, and relevant
antibacterial and photothermal properties have led to extensive applications of conductive materials in diverse fields, such as sensors, conductors, supercapacitors, medical, tissue engineering, energy storage, and so on [51, 77-81]. However, the incorporation of the conductive substances into biomaterials remains a great challenge. Most CPs, carbon nanomaterials, metals, and metal oxides are insoluble in aqueous solution and tend to aggregate resulting in a lower conductivity [82- 85]. Thus, the homogeneous dispersion of these conductive substances requires elaborate pretreatment. Surface modification is a mature method to improve the solubility and stability of the conductive nanomaterials [86]. Hydrophilic molecules including small molecules and polymers can be grafted onto conductive nanomaterials through chemical conjugation or noncovalent interactions. Polymer wrapping assisted by strong stirring and sonication has been proved effective in promoting the even dispersion of nanosized conductive materials and preventing aggregation. Besides, functionalization of conductive polymers could be realized through doping mechanism [52].
The stability, degradability, and cytotoxicity of CPs under
physiological conditions also matter a lot [87]. Carbon nano-
materials and metal nanomaterials could not even degrade
in vivo [88]. Besides, the incorporation of conductive sub-
stances would make a great difference on the mechanical
properties of the pristine biomaterial [89]. The balance and
compromise between the conductivity, biocompatibility and
mechanical properties should be fully investigated before
in vivo application.
Excitingly, novel 2D inorganic conductive nanomaterials as black phosphorus (BP) and transition metal carbides and nitrides (MXene) have recently attracted great attention for their electroactivity and demonstrated great potential in biomedical applications for the biodegradability, photothermal effect, and antibacterial activities [90- 94]. On the other hand, their applications are hindered by poor stability under
ambient conditions or in liquid medium [81, 95]. The applications of BP and MXene in wound healing are still in the very preliminary stage while facing plenty of challenges and new opportunities. In this context, these conductive biomaterials are reviewed in two categories, 2D and 3D conductive biomaterials. In addition, we focus on providing brief guide to the fabrication methodology for incorporating conductive materials into wound dressings and scaffolds.
3 2D Conductive Biomaterials for Wound Healing
3.1 Film
Thin film is semipermeable, allowing oxygen and water vapor to pass through, while a large amount of fluids and bacteria are blocked. Due to the flexibility, lightweight, and poor water-uptake capacity, film-based wound dressing is suitable for delicate skin and thin wounds with little exudate [96]. Meanwhile, the film should not be too compact, because low vapor transmission and oxygen permeability would deteriorate wounds resulting from moisture accumulation and skin maceration. Thin films can also serve as matrices for bioactive reagents, including conductive materials. Due to ease handling both for in vitro and in vivo, conductive thin films have been widely employed to study the mechanism of how conductive materials modulating cell activities, which also facilitates their further applications in other more sophisticated structures [97]. Thus, conductive film-based wound dressings have attracted great attention, and many studies focused on the mechanism of conductive substances promoting wound healing. Since cells were cultured on the surfaces of these films, the vapor transmission and oxygen permeability of these films were not taken into consideration [98-100].
Films are usually fabricated via a solvent casting method [97, 100, 101]. Thus, compared with carbon nanomaterials that tend to aggregate and need assistance from other amphipathic polymers or technology to form monodispersion, CPs synthesized via polymerization through solventsoluble monomers demonstrate tremendous advantages in designing conductive films [102, 103]. Table 1 lists previously reported conductive films as wound dressings.
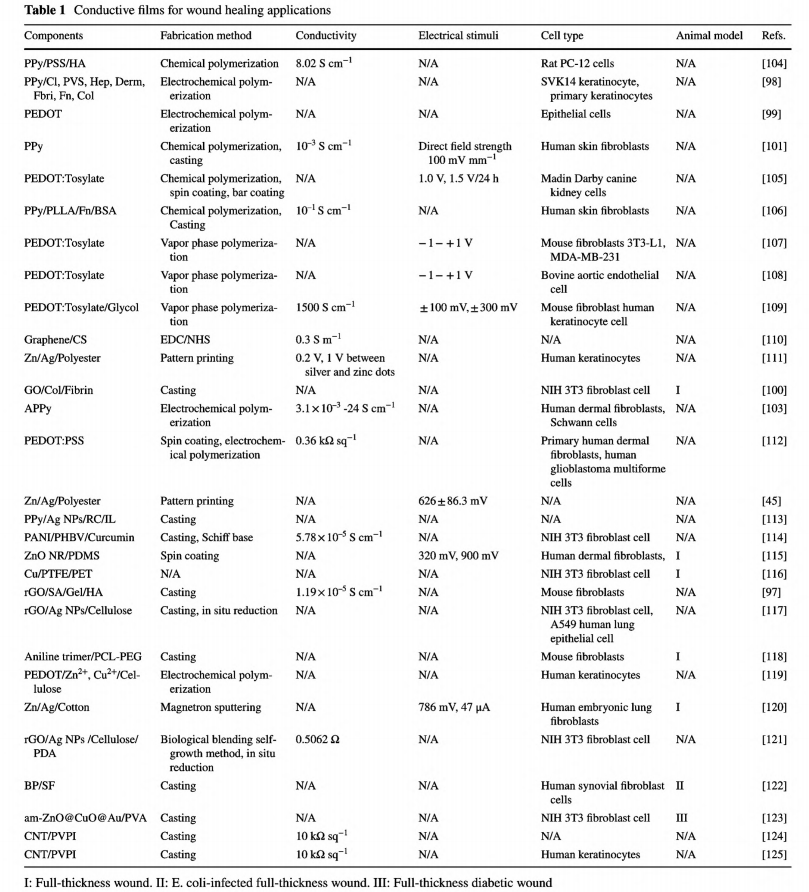
Polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), and polyaniline (PANI) and its oligomers are mostly used for their biocompatibility, facile synthesis, and tunable conductivity generated by various dopants [52, 82,102]. Even though the pure CP films could be fabricated through electrochemical polymerization with controlled morphology, the inherent brittleness, mechanical rigidity, and nondegradability restrict their applications as wound dressings [79]. Simple blending or conjugation with other elastic biocompatible polymers is a general principle to avoid these limitations [26, 37, 61].
3.1.1 PPy
Companying with the surging of ES enhanced wound healing, conductive films have been used as electrodes for the ES devices. PPy-based polymer films have been detailedly explored. Shi group have synthesized PPy nanoparticles via a water-in-oil emulsion system and incorporated PPy into PLLA [101]. This conductive composite film could support cell attachment, spreading, and proliferation of human cutaneous fibroblasts with or without ES. Besides, cell viability was much higher when the composite film was under direct electrical field strength of 100 mV mm-'. Later, they explored the detailed mechanism of PPy/PLLA film prompting wound healing. Under ES mediation (100 mV mm ), the conductive PPy/PLLA film could enhance the viability of human cutaneous fibroblasts by modulating cytokines as IL-6 and IL-8 [126]. Mahmoud et al. have fabricated heparin-doped PPy/PLA membrane [127]. When exposed to ES, the conductive film revealed enhanced dermal fibro-blast activity with higher expression of FGF-1 and FGF-2
and promoted myofibroblast transdifferentiation with higher level of a-smooth muscle actin. Later, they have proved at genetic level that PPy conductive composite film could modulate the expression of various genes in the wound healing process [128]. In addition, PPy is responsive to electrical signals, so it can be used to design electronically controlled drug delivery [129]. Justin et al. have developed an electronically controlled drug delivery system based on PPy contained conductive polymer flm promoting wound healing process [130].
Without ES, PPy-based conductive composite films also have proven positive effects on wound healing. Many studies have proved that the addition of PPy would affect the performance of the conductive films in promoting wound healing to a large extent. Collier et al. fabricated a bilayer film based on PPy doped with PSS as a thin bottom layer and PPy doped with hyaluronic acid as a thick top layer [104]. This bilayer conductive film exhibited superior electrical conductivity, electrochemical properties, surface morphology, and mechanical integrity than single layer only containing HA and PPy. As this bilayer conductive films could promote angiogenesis, the author suggested this bilayer conductive film could act as promising candidate for wound healing application.
Different dopants, including proteins and polysaccharides,can be used to fabricate PPy-contained films [98]. Among them, the film that contained dermatan sulfate revealed the largest effect on supporting keratinocyte growth. PPy nanoparticle could also be modified with fibronectin or bovine serum albumin and then deposited with PLLA to develop bioactivating PPy conductive membrane [106]. The fibronectin-modified PPy/PLLA film supported a higher adhesion and spreading of human skin fibroblasts compared with BSA-modified PPy/PLLA membrane. This was because these incorporated bioactive molecules also play a signifcant role in modulating cell activities.The hydrophilicity of film surface is another key factor that affects cellular activities [96]. The introduction of PPy would increase the hydrophobicity of the designed composites, thus generating a negative effect on cell attachment. Amine- functionalized PPy has been proved with improved hydrophilicity compared with pure PPy and demonstrated enhanced adhesion toward human fibroblasts and Schwann cells [103].
3.1.2 PEDOT
PEDOT possessing a better thermal stability and higher conductivity than PPy has also been chosen to fabricate conductive films. Firstly, the enhanced cell adhesion and proliferation of epithelial cells on PEDOT films have been convinced [99]. Besides, this PEDOT film showed a remarkable affinity toward protein as bovine serum albumin, thus demonstrating good electrocompatibility and electroactivity. The conductivity of PEDOT can be further enhanced by the introduction of dopant. Wan et al. developed a styrenesulfonate doped PEDOT conductive film named as PEDOT-TOS and combined this film with indium tin oxide assembling a redox gradient along this PEDOT-TOS stripe [107]. The PEDOT-TOS validated cell viability exceeding 98% regardless of its redox states. Besides, under gradient distributed potential, the cultured mouse fibroblasts exhibited density gradient as well, which may associate with differences in orientation of the adsorbed protein at the two electrodes or a higher concentration of adsorbed protein on the reduced side of the device would suppress cell adhesion. The cell density and distribution could be controlled by the dimensions of the conducting film and programmed applied bias. Moreover, this device could be utilized to control cell migration under electrical cues. The migration speed, direction, and directional persistence time of bovine aortic endothelial cllls can be enhanced and controlled by the applied bias, thereby indicating the potential of PEDOT-TOS film as wound dressing [108].
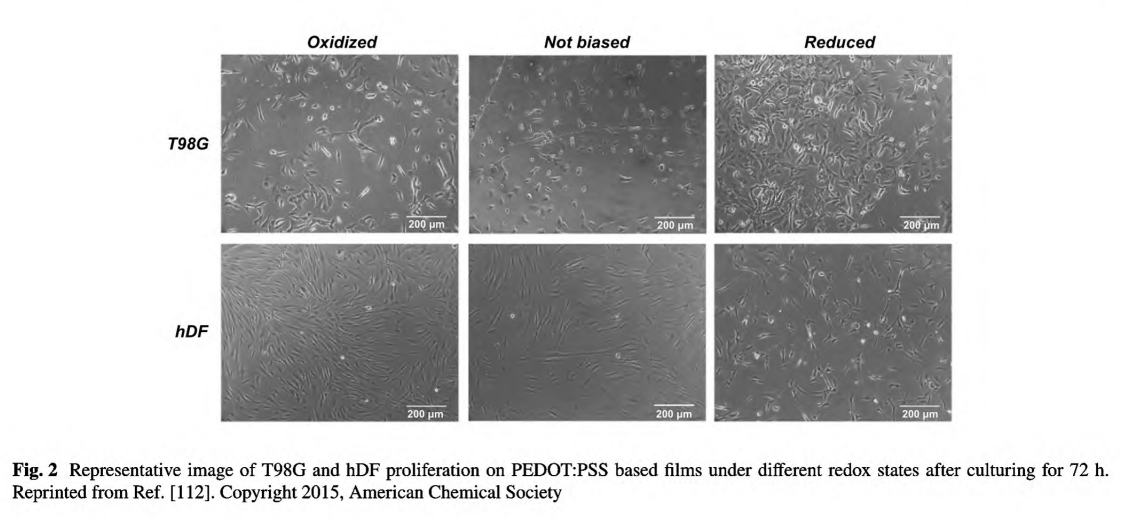
The polyelectrolyte, PSS, could not only extremely enhance the conductivity of the PEDOT system, but also result in a water-soluble polyelectrolyte system with good film-forming benefit. Marzocchi et al. have managed to deposit PEDOT:PSS on commercial film via spin coating and electrochemical polymerization and proved the films in oxidized state exhibiting enhanced proliferation rate toward hDF [112]. Meanwhile, the enhance cell growth was cell- dependent for that the enhanced growth of T98G was found on reduced substrate, as shown in Fig. 2.
Moreover, Nguyen et al. proved a polycarbonate membrane composed of PEDOT nanotubes could be utilized as a carrier for controlled ex vivo transdermal drug delivery [131]. Under programmed potential, the drug release prolife of insulin could be finely tuned, attributing to the electroresponsive dynamic electrostatic interaction between drugs and PPy nanotube. In summary, PEDOT-based films possess multiple advantages for wound healing application.
3.1.3 PANI and Aniline Oligomers
Although being one of the most widely used CPs and possessing good chemical and mechanical stability, there are rare reports about pure PANI-based conductive films as wound dressing, probably due to its poor solubility and high infusibility. It is hard to dissolve PANI under mild conditions and further form a thin film [132]. On the other hand, the polymerization of aniline monomers requires an acidic
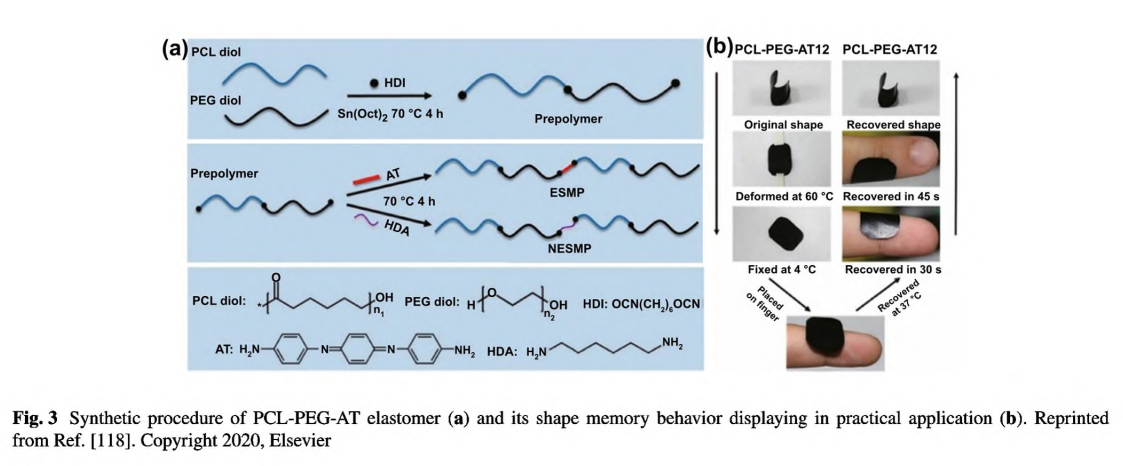
condition (pH< < 4) which may be severe to other biomolecules and restrict its direct implementation [82]. To circumvent this issue, Pramanik et al. developed a facile strategy to conjugate PANI nanofibers on the aminated PHBV surface (PHBV-g-PANI) in a methylsufinyl carbanion-treated DMSO solution [114]. Through π-π interactions, curcumin could be entrapped by PANI; thus, this hydrophobic drug was loaded within PHBV-g- PANI. This curcumin-loaded conductive film composite exhibited antibacterial activities and could promote cell migration and proliferation on the injured tissues. However, PANI still exert cytotoxic effect on cells and could not degrade in vivo. In stark contrast, aniline oligomers with well-defined structure, good solubility, biocompatibility, and similar electroactivity have been used to fabricate conductive films in biomedicine and tissue engineering [51]. Our group designed a series of conductive polyurethane- -urea elastomer films composed of PCL segment, PEG segment, and aniline trimer [118]. As shown in Fig. 3a, this elastomer adopted a delicate design strategy: the PCL segment provided good mechanical properties, the PEG segment endowed the film with appropriate hydrophilicity and swelling ratio, and aniline trimer ensured electroactivity and antioxidant activity. More impressively, this polyurethane-urea film exhibited shape memory properties (Fig. 3b). Therefore, this film could be manufactured into specific shape suitable for wounds in cylindrical body. When the film in a temporary shape was applied on the wound site, it would rapidly restore to the original shape, thereby generating a force which could facilitate wound closure. In a full-thickness skin wound assay, this conductive elastomer film demonstrated significantly enhanced wound healing performance with the highest collagen deposition and granulation tissue thickness than no-electroactive film and commercial dressing (Tegaderm™). To this end, we suggested that the shape memory properties should be emphasized in wound healing applications as well as its well-established application in minimally invasive surgery, because it could maintain the original shape independent of external force, thereby promoting wound contraction during the initial stage of wound healing.
3.1.4 Carbon Nanomaterials
Carbon nanotube (CNT) in single-walled and multi-walled forms has been comprehensively studied and applied in various fields. It is well established that CNT has large specific surface area, electrical conductivity, good mechanical properties, and spectroscopic properties. CNT also demonstrates promising properties in skin tissue engineering, including antibacterial activities, high drug loading efficacy, angiogenesis enhancement, and tissue repair-related gene expression manipulation [85, 133]. However, the cytotoxicity of CNT needs to be fully evaluated when facing the application of direct contact to human cells, considering the current reports that the toxicity of CNT depends on various parameters, including length, diameter, impurities, and modified agents [54, 85].
Although the incorporation of CNT into polymeric matrix endows the composites with enhanced mechanical and physiochemical properties, the incorporation process suffers from the strong tendency to agglomerate and hydrophobic nature of CNT. Polymer wrapping technique is a classical effective method to solubilize CNT [134]. Simmons and his colleagues reported an antiseptic film based on CNT and polyvinylpyrrolidone-iodine, in which CNT was solubilized by polyvinylpyrrolidone-iodine and encased in a polymer monolayer with a helical coil conformation [124, 125]. Polyvinylpyrrolidone-iodine with slow release of iodine also provided this film with antiseptic and antibacterial properties. Combining the flexibility, oxygen permeability, high bacterial killing efficacy, biocompatibility, and conductivity (10 kΩ sq-1), the author suggested this film with promise as bandage for wound healing application. However, according to cell viability assay on human keratinocytes, the application of this film meets limitation. Iodine-containing films
were suitable for severely infected wound but should be limited in the wounds that require rapid proliferation of keratinocytes and other mammalian cells.
Graphene possessing excellent conductivity at room temperature than any other carbon materials, a high optical absorptivity, high thermal conductivity, and high mechanical strength has great value in many fields [135]. However, graphene tends to aggregate, and the poor dispersion extremely restricts its application, especially as biomaterials. Graphene oxide (GO) and reduced graphene oxide (rGO) are covalently functionalized graphene which have been mostly studied [5, 53]. A large number of oxygen-containing groups as hydroxyl, epoxy, and carboxyl group supports GO with good dispersion. However, the covalent functionalization of carbon atom converting the planar sp2 hybridization to a tetrahedral sp3 hybridization would largely reduce the conductivity compared with graphene. For example, Shahmoradi et al. reported a graphene/silver incorporated wound dressing, only taking the advantages of reinforced mechanical properties of GO and its synergistic antibacterial effect with Ag NPs, without considering the electroactivity [136]. rGO containing few unreduced, covalently bonded oxy groups has a relative higher conductivity than GO [53]. Aycan et al. designed a conductive polymeric film consisting of sodium alginate, gelatin, hyaluronic acid and rGO [97]. The homogeneous rGO suspension was formed after ultrasonic treatment. rGO was incorporated into the polymeric network through strong Van der Waals interaction and hydrogen bonding. The conductive film demonstrated enhanced mechanical properties, good biocompatibility, adequate water vapor transmission rate, and improved oxygen permeability. Moreover, this conductive film was loaded with an anti-inflammatory drug ibuprofen and demonstrated a controlled release profile. Therefore, the author suggested this conductive film has great potential in wound dressing. Khamrai et al. synthesized a cellulose/rGO/Ag NPs composite film as multifunctional dressing with conductivity, and antimicrobial activity [117]. Notably, as the cellulose was modified with dopamine, this film exhibited an adhesive nature which could facilitate the adhesion and proliferation of NIH 3T3 fibroblast cell. A multifunctional composite film based on similar components was developed by Zhang et al. [121]. The difference is that polydopamine was utilized as a ligand to prevent Ag NPs agglomeration and silver loss.
3.1.5 Metals and Metal Oxides
Ag and Zn dots printed on polyester sheets or cotton nonwovens could work as bioelectronic wound dressings and have already proven significant effect on the improvement of human keratinocyte, cellular behavior activation, and wound healing by generating sustained microelectric potentials and restoring disrupted physiologic bioelectric signals on wound sites, while the voltage was depended on the size of the dots and the distances between metal dots [45, 11, 120].
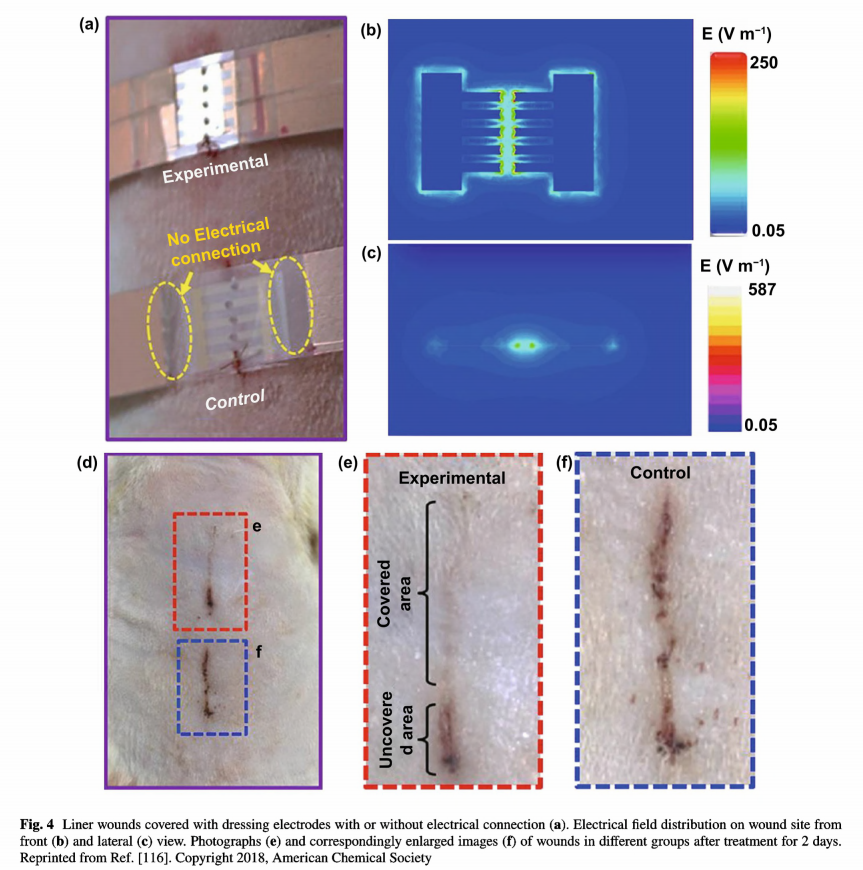
As mentioned above, the application of electrotherapy generally requires extracorporeal power supplies, which is not convenient for patients. Recently, the innovation of triboelectric nanogenerators that could harvest biomechanical energy and further generate periodic ES has expanded the development of facile electrotherapy [78, 137]. Zinc oxide nanorods can be integrated onto PDMS film assembling a highly bendable, stretchable piezoelectric dermal patch [115]. Since a piezoelectric potential was generated upon mechanical deformation induced by animal motion, the wound was under continuous stable ES. The patch displayed therapeutic effect to wounds and promoted cellular metabolism, migration, and protein synthesis. Fu et al. developed an efficient electrical bandage based on Cu foil as the electrodes, and polytetrafluoroethylene as the triboelectric active layer, and polyethylene terephthalate as the static substrate [116]. The bandage had good adaptability to soft skin. When the rat was equipped with a bandage around the chest, an alternating discrete electric field was generated from rat breathing. As shown in Fig. 4, liner wound covered with the bandage which connected with nanogenerator was under a strong and localized electrical field. Compared with the unclosed wound in control group, wound under electric field nearly fully closed after wearing the bandage for 2 days. From in vivo animal assay on large rectangular
wounds, wounds covered with the bandage complete wound contraction within 3 days, much rapid than the wounds wearing the same device but without electrical connection.
3.1.6 Other 2D Inorganic Nanomaterials
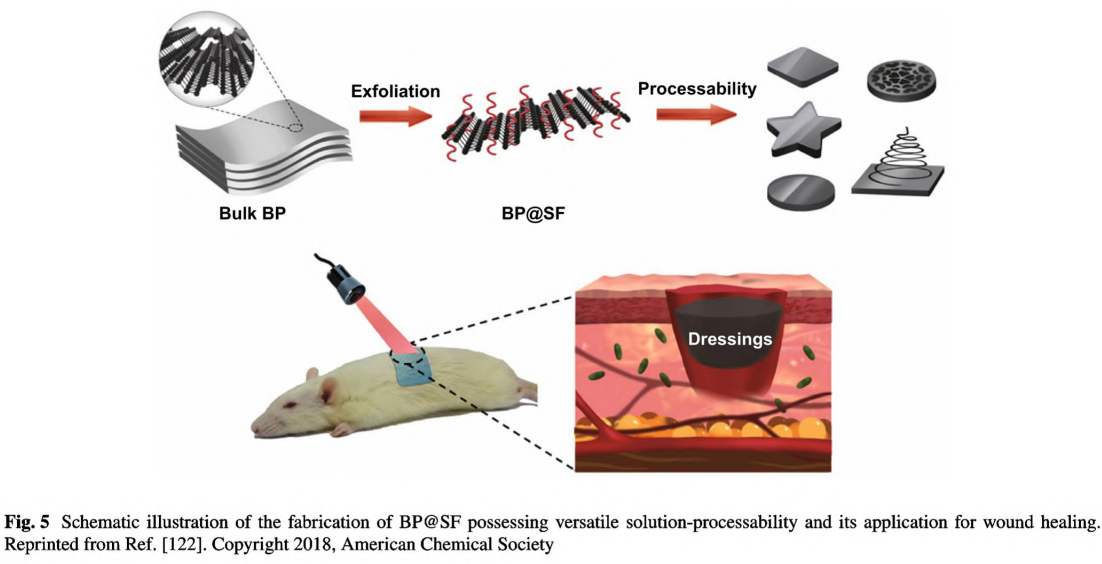
Black phosphorus (BP) as a 2D layered semiconductor material demonstrates novel electrical and optical properties including excellent charge carrier mobility, tunable bandgap, and remarkable photothermal conversion efficiency [81]. Considering the excellent photothermal effect [91] and biocompatibility [90], BP has been regarded as a promising candidate for biomedical applications [91], such as cancer therapy [138], antibacterial therapy [139]. and wound healing [94]. However, BP nanosheets are commonly synthesized via liquid exfoliation with organic solvents and could not stand a long-term usage because they would degrade rapidly under the physiological environment. In 2018, Huang et al. employed SF as the exfoliating agent to fabricate BP nanosheets via liquid exfoliation method in aqueous solutions as shown in Fig. 5 [122]. The BP hybrid nanosheets(BP@ SF) demonstrated long- term stability, excellent photothermal antibacterial activities, and enhanced wound healing performance. Notably, due to the assistance of SF, BP can be easily fabricated into various forms, including fiber, film, and sponge, thus benefiting further applications in diverse types of wounds. BP can be more easily incorporated into hydrogels, nanofibers and other scaffolds by sonication or electrostatic absorption [81]. More importantly, due to remarkable photothermal effect, BP-based conductive biomaterials can be used for combinational photothermal therapy and wound healing [140].
Another superstar of 2D inorganic nanomaterials in biomedical field is MXene, which has been applied in biosensors, supercapacitors, batteries, catalysis, and therapeutics [93]. Similar to BP, stability under physiological conditions is one severe issue that restricts the biomedical application of MXene [95]. The application of MXene in wound healing is in the preliminary stage. Even though some reports have validated its positive performance in promoting wound healing [141-143], there is no report about MXene-based conductive film as wound dressing yet.
3.2 Membrane
Membrane dressing is a semipermeable biomaterial similar as thin film, except that membrane has a certain degree of water absorption capacity, and thus can be used on wounds with moderate exudate, such as superficial wounds, frictional wounds, scratching wounds, and skin donor sites [144,145]. Gharibi et al. developed an aniline tetramer embedded polyurethane/siloxane membrane as intelligent wound dressing [146]. The introduction of inorganic siloxane moieties endowed the hydrated membrane with enhanced mechanical properties, because hydrolysis and condensation of methoxysilane moieties would display a reinforcement effect on the polymeric network [147]. It is worth to mention that after being crosslinked by siloxane moieties, doped with camphorsulfonic acid and further solvent extraction/purification, the membrane should be immersed into distilled water maintaining in a hydrated state. Compared with commercial polyurethane-based dressing with compact structure, these membranes exhibited improved water absorption and higher oxygen and water vapor transmission rate. Furthermore, Ag NPs were incorporated via in situ reduction by the embedded oligoaniline, endowing the membranes with antibacterial activities and enhanced conductivity. Therefore, these membranes demonstrated promising radical scavenging property, perfect antimicrobial activity, and improved fibroblast cells viability and proliferation, all indicating great potential as wound dressing. In their follow-up work,a series of polyurethane/siloxane-based conductive dressing membranes composed of castor oil, ricinoleic methyl ester and aniline tetramer with conductivity, high biocompatibility, remarkable analgesic and anti-infammatory effects, and promotion on epithelialization were developed [148]. The conductive membranes and nonconductive membranes all demonstrated significantly enhanced wound healing performance than sterile cotton gauze in terms of rapid wound closure, completc re-epithelization of the wound, enhanced vascularization, and collagen deposition. Commercial membrane modified with conductive component is another simple approach to fabricate conductive membrane. Polyethylenimine conjugated PPy nanopigments can be coated on a PE-fiber-constructed membrane through dip coating method [149]. This conductive membrane demonstrated excellent photothermal convertible performance, antibacterial activities, warming preservation, and anti-vaso-constriction in vivo.
3.3 Micro- and Nanofibers
Micro- and nanofibers have great potential in tissue engineering for their fibrillar architectural resemblance to ECM [26]. The porous structure and high specific surface area grant oxygen permeation, nutrient exchange, and management of relatively large exudate through absorbency [150]. In addition, the fibers are semipermeable, and the smallsized pores could restrain the penetration of pathogens. Commonly, micro- and nanofibers-based wound dressings are soft and compliant, thus can be easily applied on wound sites, and retain conformity under human movement [74]. Moreover, fibers could also be assembled into any desired shape to accommodate any shaped wounds. Table 2 presents a list of micro- and nanofibers as wound dressings.
One approach to produce conductive fibers is to coat the fibers with conducting polymers. Wang et al. employed such a method to fabricate conductive PET microfibers [155]. Thin and uniform PPy was deposited on the surface of the commercial PET microfibers without blocking inter- fiber space. Under pulsed ES of 5 V, these conductive fabrics exhibited enough conductivity and stability within 24 h to
promote fibroblast growth.
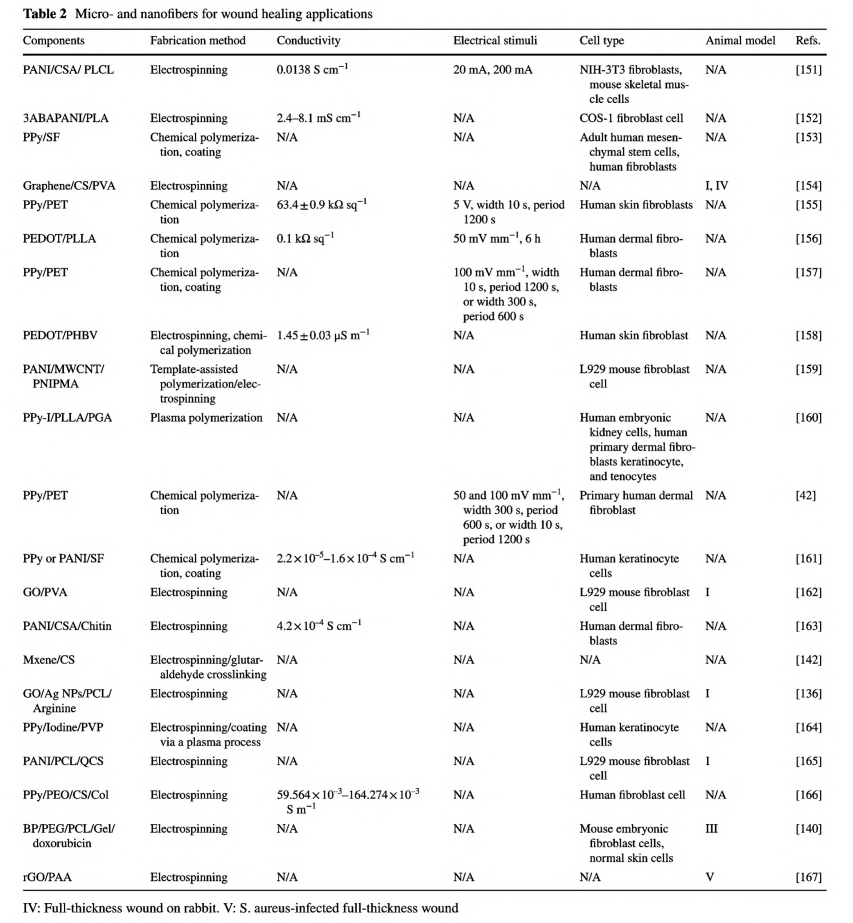
Degummed silk fibroin (SF) is a natural protein fiber and has been extensively used as wound dressing [70]. GH et al. developed a simple strategy of in situ polymerization of pyrrole and aniline on SF fibers [161]. PPy and PANI were coated on SF by forming interactions with peptide linkages while mostly preserving protein assembly. Compared with the smooth surface of the primitive SF fibers, some particles and aggregates were found on the coated fibers, which would facilitate cell adhesion. As the conductivity of PPy/SF and PANI/SF fibers was at the same magnitude as the corresponding conjugated CPs, it suggested that this coating strategy was effective to construct homogenous and continuous conductive pathways on the surface of SF fibers. Compared with SF fibers, these two kinds of conductive SF fibers demonstrated positive effects on human immortalized keratinocytes adhesion and proliferation. Recently, Jia et al. reported conductive SF microfibers integrated bioelectronic with excellent conductivity for wound healing and diagnosis in diabetes. As shown in Fig. 6, the novelty of this work was the protection and pre-modification of SF microfibers with a PDA layer, which assisted the assembly and uniform deposition of PEDOT, as well as a well-connected conductive pathway.
However, this strategy is limited to polymers with a natural fibrillar structure or commercial fabrics products, and the diameter of these fibers cannot be adjusted. It is still of great necessity to develop fibers with tunable diameter ranging from nanometer to micrometer utilizing different polymers.
Electrospinning is a straightforward method to produce ultra-fine nanofibers or nanofiber assemblies with narrow diameter distribution [26]. Two general approaches have been developed to fabricate conductive electrospun nanofibers. The first one is the above-mentioned coating strategy. Cervantes et al. fabricated electrospun SF micro- and nanofibers with methanol treatment and then coated with PPy [153]. This PPy/SF conductive mat could promote human fibroblasts adhesion and proliferation. Besides, the diameter of SF fibers fabricated through electrospinning was much smaller compared with the SF fibers isolated from wild cocoons [161]. Due to its good solubility in water,PEDOT: PSS is suitable for this coating strategy. Chang et al. fabricated a polylactide and poly(3-hydroxybutyrateco-3-hydroxyvalerate) electrospun membrane and then dipped this membrane in PEDOT: PSS solution. Notably, no change was found on the morphology of the membranes after coating treatment, while the wettability and surface roughness increased with the increase of PEDOT:PSS content that could faciltate cell adhesion, cell proliferation, and cell-scaffold interactions [158].
Another classic method to fabricate conductive fibers is direct electrospinning of conductive materials/polymer composites. The limiting factor may be the poor solubility or dispersion of these conductive materials. Zhang et al. managed to fabricate GO-modified electrospun polyvinyl alcohol nanofibers, and ultrasonication for 30 min was necessary for the homogenous dispersion of GO [162]. This conductive nanofibrous scaffold could promote growth and adhesion of L929 fibroblasts, because PVA supported excellent water absorption ability, biocompatibility, and physiological stability, and GO provided enhanced mechanical properties, electroactivity, and protein affinity. Graphene can be homog-enously dispersed with PVA in DMF/H2O mixed solvent after ultrasound stirring treatment for 30 min. The designed nanofibers exhibited excellent antibacterial activities and enhanced wound healing effect [154].
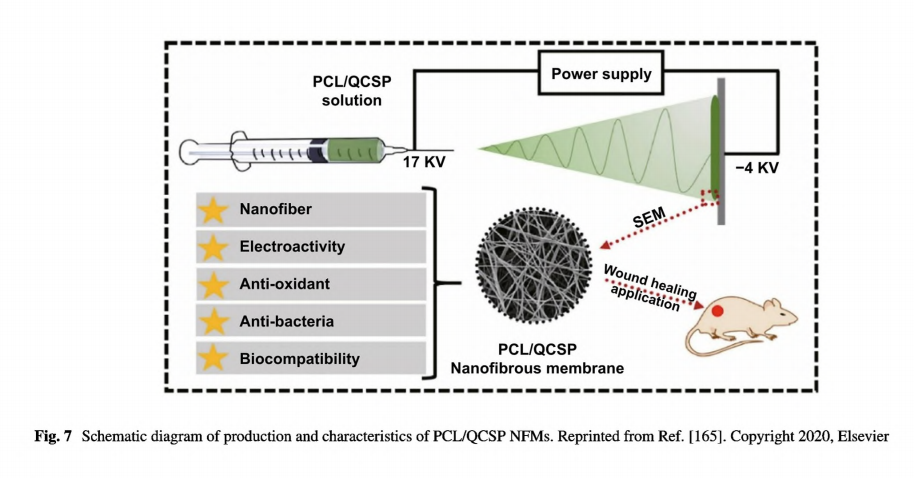
The insolubility of PANI in most solvents limits its application in direct electrospinning. Even though there are reports about fabricating PANI incorporated nanofibers by direct electrospinning and validating their feasibility in skin tissue engineering, either a long- term pretreatment [163] or a low concentration of PANI solution was required [151]. Nikolaidis et al. synthesized a poly(aniline-co-3-aminoben-zoic acid) copolymer with improved solubility in basic aqueous media and polar solvents which benefited further electrospinning [152]. Poly(aniline co- 3- -aminobenzoic acid) and PLA blends could be well dissolved in dimethyl sulfoxide/tetrahydrofuran mixture. The corresponding nanofibrous mats doped with HCI have been confirmed as promising antimicrobial dressings owing to the conductivity, antimicrobial capability against Staphylococcus aureus, and enhanced COS-1 fibroblast proliferation. Recently, our group has developed a series of electroactive nanofibrous membranes (PCL/QCSP NFMs) based on quaternized chitosan grafted polyaniline (QCSP) and PCL, as illustrated in Fig. 7 [165]. In this membrane, QSCP segments provided multiple functions as excellent electrical conductivity, good solubility, antioxidant, and antibacterial activity, while PCL segments ensured the membranes with suitable mechanical properties. Results from in vitro cell culture and in vivo wound healing test and histological analysis have all confirmed that PCL/QCSP NFMs could promote cell proliferation, collagen deposition, and angiogenesis and accelerate wound healing process. Overall, conductive micro- and nanofibers can be fabricated through facile methods and promote wound healing effectively with good compliance to skin, easy removal, and suitable mechanical properties.
The literature is to be continued……
This article is excerpted from the Nano-Micro Letters by Wound World.