1. Introduction
Diabetes Mellitus has become a major healthcare issue for the Western world over the years. Of particular concern is the decreasing age of patients at diagnosis, which has placed the disease in the center of attention of the scientific community due to possible long-term health problems for those with early onset diabetes.
In recent decades diabetes mellitus has started to affect more men during their reproductive years [1] [2] and various studies have been proposed to explain the phenomenon [3] [4]. Of these, diminished male fertility in particular decreased sperm counts and quality has received significant attention [5].
The disease can exhibit male reproduction function at multiple levels especially spermatogenesis as it is under endocrine control and therefore much attention and many scientific experiments focus on Diabetes Mellitus. Diabetes Mellitus is a chronic disease which arises either due to the pancreas which does not produce enough insulin or due to the body which cannot use effectively the insulin it produces.
2. AGE—Their Relation to DM
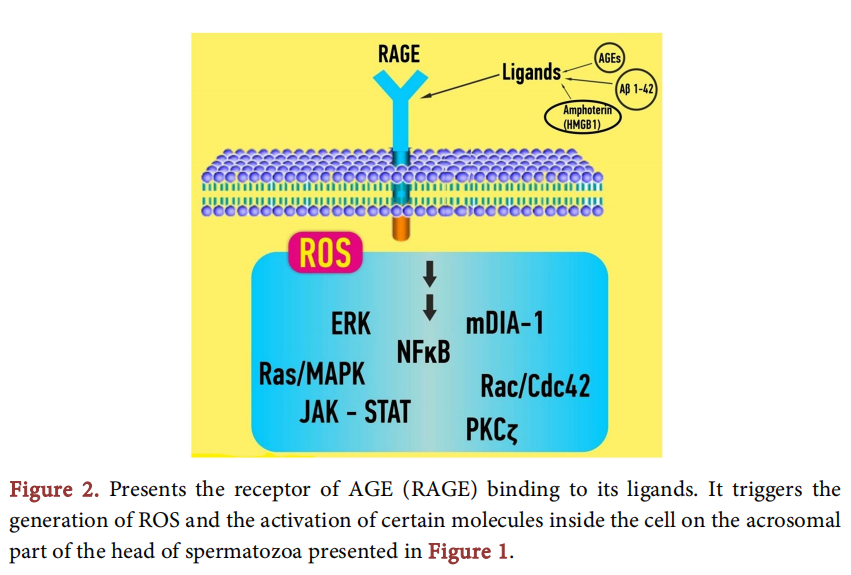
One of the factors, which received much attention over the years is the formation of some products in non-enzymatic reactions between sugar and amino groups of proteins, lipids and DNA in hyperglycemic conditions [6] [7]. Those products, under the name Advanced Glycation End products or AGE’s, deploy a diverse array which consists of fluorescent and non fluorescent species and are reported to exhibit a key role in initiation and progression of many diabetic complications [7] [8]. [9] showed an increased level of N-Carboxyl methyl-lysine (CML), the best known and the most prominent AGE, in the reproductive tract of diabetic men and [10], using spectrofluorimetric detection, found that total AGEs, when measured in seminal plasma of diabetic men were significantly higher than in non-diabetic group. AGEs can alter the function of macromolecules by modifying their structure or by generating reactive oxygen species (ROS) via their receptor [11]. The receptor for advanced glycation end products is a multiligant cell receptor, member of the immunoglobulin superfamily. Ligands for this receptor are AGE’s, as well as amphoteric tranthyretin, β-amyloid and calgranulin which engage the extracellular domain [12] and lead to interactions that activate cell signaling cascades which control several genes through NF-KB factor. [12] have reported causative effect in many diabetic complications generated by those interactions.
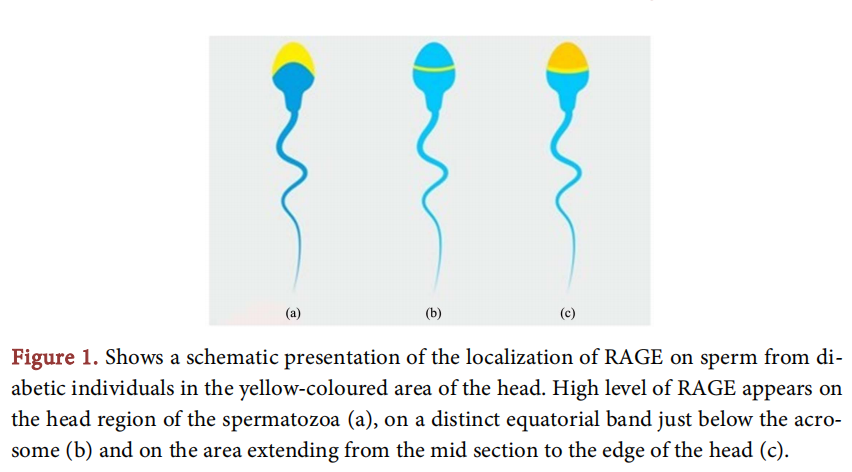
The receptor for AGE’s, RAGE, is a member of the immunoglobulin superfamily of cell surface molecules. It is composed of an extracellular region containing a single “V”-type Ig domain and two “C”-type Ig domains followed by a hydrophobic transmembrane domain and a short, highly charged cytosolic domain which is essential for the cellular effects mediated by ligand-RAGE binding [13]. The extracellular region confers ligand-binding properties, probably via the “V” domain, and recognizes tertiary structures not specific amino acid sequences. As such it is a pattern recognition receptor able to engage classes rather than individual molecules [14]. Once ligand-RAGE binding occurs, it perturbs cellular properties and sets the stage for the sequelae of AGE generation/accumulation [15]. [16] used the ELISA method to quantify the RAGE protein detected in the sperm and seminal plasma in diabetic and non-diabetic men, and found that significantly more of the sperm from diabetic men express RAGE compared with sperm from healthy donors. This finding is probably a result of the heightened rate of AGE accumulation observed in hyperglycaemic conditions. RAGE found to be expressed on the acrosomal cap and the equatorial region of the sperm head. The findings of this study are compatible with a newer study of 17 who also exhibited high expression of RAGE protein in spermatozoa of patients with diabetes. RAGE levels are dictated by the accumulation of its ligands. In diabetes, where hyperglycemia triggers accelerated formation and deposition of AGEs, there is enhanced expression of RAGE. However, it is also found in low levels even in normal tissue [13].
Localization and Quantification of RAGE
In order to assess the specific localization of RAGE in the reproductive tract of diabetic men scientists have conducted experiments using immunohistochemistry which result in excess radioactivity in the head region of sperm primarily, in both diabetic and non-diabetic men, especially from the midsection of the head to the very edge of the sperm, the extent of the acrosomal cap. In the testis, a uniform staining pattern appeared throughout the cytoplasm of all cell types within the seminiferous epithelium, as well as in the cytoplasm of the particular cells. The Sertoli cells, germ cells (of all developmental stages), myoid peritubular cells and Leydic cells did not exhibit any staining appearance in their nucleus [16]. When the amount(1 of RAGE was assessed, by the ELISA method [16] [17] and western blotting [17] it was measured at much higher level in diabetics compared with non-diabetic men. Those findings contradict the standard semen parameters, which show no significant difference in diabetic and non-diabetic subjects an evidence that implies that conventional sperm parameters cannot determine the capacity of spermatozoa in fertilization [18] (Figure 1).
3. ROS and Sperm Function in Diabetic Men
Oxidative stress has received much attention over the years about whether and how much it affects diabetes mellitus, whether it is a causative or a contributory factor to the disease. Reactive oxygen species (ROS) which can be a product of AGE’s, when appear in high levels in the semen, lead to oxidative stress. Oxidative stress, has long been believed that influences the fertilizing ability of the sperm [19], by producing lipid peroxides from unsaturated fatty acids which appear in high amounts in the cell membrane phospolipids of the sperm [20].
Sperm are susceptible to attack by reactive oxygen species (ROS) due to their high unsaturated fatty acid content and the absence of DNA repair mechanisms [21]. Advanced glycation end products (AGEs) have been implicated in an increasing number of diabetic complications that result from, and in, oxidative damage due to ROS generation [22] [23]. [24] studied ROS concentrations in the semen of diabetic men by using 20, 70-Dichlorodihydrofluorescein (DCDHF) which detects the production of intracellular ROS via oxidation to their respective fluorescent products. Intracellular ROS concentrations were monitored by fluorescence microscopy and flow cytometry. The results showed that concentrations of ROS in sperm fractions from men with diabetes type I were increased and in diabetes type II were significantly elevated when compared with healthy donors (Figure 2).
3.1. Lipid Peroxidation
ROS produce lipid peroxides from unsaturated fatty acids which can be found in high amount in sperm cell membrane phospholipids [20]. In order to evaluate the lipid peroxide content in spermatozoa and seminal plasma, the MDA assay is used, which means that lipid peroxidation is measured as TBARS (Thiobarbiuric Acid Reactive Substances) [17]. The method is based on the measurement of MDA (the production of malondialdehyde) spectroflurometrically. The lipid content, in both the spermatozoa and the plasma of semen, is significantly increased in diabetic men in comparison with non-diabetic subjects. As both groups had normal standard sperm characteristics, the finding could have important diagnostic and prognostic value.
3.2. Total Antioxidant Capacity
Seminal plasma has important antioxidant systems including enzymatic and nonenzymatic antioxidants that can provide the spermatozoa with a protective environment against oxidative stress [18]. [17] demonstrated that diabetic men have significantly lower seminal TAC levels compare to non-diabetic men. Total antioxidant capacity when reduced has effective role in male infertility and in diabetic cases TAC is accompanied by higher levels of MDA which means higher amount of ROS [19].
3.3. Disrupted TMP and Activated Caspase 3
In order to clarify the association between apoptosis, ROS concentration and DNA fragmentation [24] conducted a study in which semen samples from healthy donors were compared with those with diabetic donors on subcellular level. Fragmented DNA, sperm intracellular ROS and integrity of transmembrane mitochondrial potential (TMP) were investigated. The results of this study demonstrated that apoptosis (measured with disrupted TMP and activated caspase 3) increased intracellular ROS and levels of sperm DNA fragmentation, factors that seemed to be in positive correlation with each other, offer us a better insight into molecular level of the diabetic germ cells.
4. Molecular Basis
4.1. Fragmentation of Nuclear DNA—Infertility
[17] were the first to demonstrate a correlation between RAGE levels and DNA fragmentation. This result can be attributed to increased levels of AGE’s in hyperglycemic situations, as expression of RAGE is ligand dependent. When AGE’s bind to RAGE, the expression of RAGE is enhanced as well as the effects of RAGE signaling [25]. Apart from that, intense study has been conducted by scientists on the field of DNA damage in hyperglycemic conditions, as they try to find a reliable marker for determining the function of the sperm [26] [27] [28]. Many studies have determined sperm nuclear DNA fragmentation status to show significant differences at the molecular level. For the evaluation of DNA fragmentation in diabetic men some scientists have used different methods. [1] used the alkaline comet assay, while [17] and [24] used the TUNEL method. These studies have demonstrated that DNA fragmentation in the nuclei of the sperm cells of diabetic donors correlates with male infertility or subfertility and could be a causative factor for spontaneous abortion, increased rates of embryonic failure and health of next generation [17]. So, diabetes disease could be one explanation for the infertility observed in diabetic men, because studies have shown that spermatozoa of infertile men have high DNA fragmentation and there is also evidence that rate of pregnancy is reduced when semen samples show 30% DNA fragmentation in spermatozoa [27] [29] [30].
In the study of [24] DNA fragmentation was also examined and proved to be much elevated in sperm fractions of diabetes II and II than the healthy donors. In this study a strong correlation was shown between apoptosis’s signs, DNA fragmentation and intracellular ROS. DNA fragmentation and ROS concentrations were positively associated.
High levels of RAGE in spermatozoa of diabetic men are highly correlated with DNA fragmentation because activation of RAGE increases ROS production and high levels of ROS can induce DNA fragmentation [17].
4.2. Sperm Structure and Motility
In men affected by insulin dependent diabetes, sperm structure and sperm motility exhibit altered behavior. [31] investigated those two characteristics of the sperm in hyperglycemic conditions by the transmission electron microscopy (TEM) procedure. TEM revealed that sperm of diabetics is so much affected, that a diverse array of abnormalities has been detected. Acrosomes showed abnormal shape in 78%, reduced dimension in 57% of the sperm, nuclear shape was abnormal in 74% of the cases and incorrectly assembled mitochondria were present in 45% of the sperm. Finally the plasma membrane was broken in 32% of germinal cells suggesting that D.M. concludes to severe structural defects i.e. apoptosis and immaturity related defects.
4.3. Role of Insulin and Carbohydrate Metabolism in Spermatogenesis in Sertoli Cells
The above mentioned assay of [31] concluded that the role of insulin and carbohydrate metabolism in spermatogenesis is more than important as insulin seems to play role in the maintenance of spermatogenesis and testicular endocrine function. The Sertoli cells (SCs) located within the semeniferous epithelium which creates the blood testis barrier responsible for the specific microenviroment for post meiotic germ cell development, supply the physical and nutritional support for germ cells. The developing germ cells, are unable to use glucose for energy metabolism and use lactate as a substrate for ATP production [32] [33] [34] which Sertoli cells provide them. Sertoli cells prefer glucose for their metabolism, but in the absence of glucose, they metabolize other substrates such as fatty acids and ketone bodies [35]. [36] observed that human Sertoli cells do produce lactate in absence of insulin, but also acetate for the developing germ cells, as it is known that lactate is the preferred substrate for round spermatids [33] and spermatocytes [32]. Moreover on the topic, [37] demonstrated that hSCs, when cultured in insulin deprivation conditions, expressed the ability to adapt their glucose metabolism by modulating the expression of GLUT1 and GLUT 3 m-RNA level and decreasing significantly GLUT 3 m-RNA levels. GLUT are hexose transporters on the plasma membrane of Sertoli cells and through them the SCs take up glucose from the external medium [38]. The action of those transporters is usually the rate limiting step for glucose metabolism in the cells [39]. Studies in streptozotocin induced diabetic rats showed severe dysfunction of the reproductive tract, because diabetic rats presented decreased reproductive organ weights as well as diminished sperm counts and motility [40]. In a study conducted in 2007 by [41], showed that STZ induced diabetic mice demonstrated significant oxidative stress during early diabetic phase, a finding that could contribute to the development of testicular dysfunction which could lead to altered steroidogenesis and impaired spermatogenesis, which implies that insulin seems to control or take part in control of spermatogenesis and sperm maturation.
4.4. DNA Gene Expression
The importance of mt-DNA quality in male fertility has been under investigation [41] [42] and deletions in mt-DNA were associated with sperm motility and infertility [43] [44] [45]. Sperm is transcriptionally and translationally silent, but it also contains a complex population of mRNAs [46] with an archive of gene expression [47] [48] [49]. Mt-DNA is subject to much greater oxidative stress than n-DNA, because it relies close to respiratory chain complexes, which produce reactive oxygen species as a by-product of oxidative phosphorylation [50], but it also lacks histone protection [51] which makes it more vulnerable. Mutations 10 - 100 times higher than those in n-DNA arise from rapid replication, inefficient proof reading and limited repair mechanisms [44]. Damage to mt-DNA in sperm has been shown to occur at much lower levels of oxidative stress than n-DNA [52] reinforcing its importance as a sensitive indicator of sperm health [53].
5. Conclusion
In conclusion, Diabetes Mellitus affects the reproductive function of diabetic men on a subcellular level and many scientific studies confirm this hypothesis. Due to hyperglycaemia, the organism is circumstanced such that it exhibits, among others, excess DNA damage, elevated RAGE concentration due to AGE’s accumulation and a diverse array of sperm abnormalities. All those observations conclude the point that the subcellular parameters seem to be causative or contributory to fertility problems as the disease progresses. Those factors, as they are in positive correlation to each other, could become, when research develops, a promising tool in hands of the scientists who search for new biomarkers, and provide new perspectives in the field of male subfertility in cases where fertility problems seem hard to be solved.
Authors’ Contributions
Investigation, writing original draft preparation AP; supervision, project administration, MT, PK. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
AP, MT and PK are not aware of any conflict of interest that may prejudice the impartiality of this article.
References
[1] Agbaje, I.M., Rogers, D.A., McVicar, C.M., McClure, N., Atkinson, A.B., Mallidis,C. and Lewis, S.E. (2007) Insulin Dependent Diabetes Mellitus: Implications for Male Reproductive Function. Human Reproduction, 22, 1871-1877. https://doi.org/10.1093/humrep/dem077
[2] Wild, S., Roglic, G., Green, A., Sicree, R. and King, H. (2004) Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care, 27, 1047-1053. https://doi.org/10.2337/diacare.27.5.1047
[3] Carlsen, E., Giwercman, A., Keiding, N. and Skakkebaek, N.E. (1992) Evidence for Decreasing Quality of Semen during Past 50 Years. British Medical Journal, 305, 609-613. https://doi.org/10.1136/bmj.305.6854.609
[4] Morgan, S.P. (2003) Is Low Fertility a Twenty-First-Century Demographic Crisis? Demography, 40, 589-603. https://doi.org/10.1353/dem.2003.0037
[5] Jensen, T.K., Carlsen, E., Jorgensen, N., Berthelsen, J.G., Keiding, N., Christensen, K., Petersen, J.H., Knudsen, L.B. and Skakkebaek, N.E. (2002) Poor Semen Quality May Contribute to Recent Decline in Fertility Rates. Human Reproduction, 17, 1437-1440. https://doi.org/10.1093/humrep/17.6.1437
[6] Singh, R., Barden, A., Mori, T. and Beilin, L. (2001) Advanced Glycation End-Products: A Review. Diabetologia, 44, 129-146. https://doi.org/10.1007/s001250051591
[7] Unoki, H., Bujo, H., Yamagishi, S., Takeuchi, M., Imaizumi, T. and Saito, Y. (2007) Advanced Glycation End Products Attenuate Cellular Insulin Sensitivity by Increasing the Generation of Intracellular Reactive Oxygen Species in Adipocytes. Diabetes Research and Clinical Practice, 76, 236-244. https://doi.org/10.1016/j.diabres.2006.09.016
[8] Mallidis, C., Agbaje, I.M., Rogers, D.A., Glenn, J.V., Pringle, R., Atkinson, A.B., Steger, K., Stitt, A.W. and McClure, N. (2009) Advanced Glycation End Products Accumulate in the Reproductive Tract of Men with Diabetes. International Journal of Andrology, 32, 295-305. https://doi.org/10.1111/j.1365-2605.2007.00849.x
[9] Karimi, J., Goodarzi, M.T., Tavilani, H., Khodadadi, I. and Amiri, I. (2011) Relationship between Advanced Glycation End Products and Increased Lipid Peroxidation in Semen of Diabetic Men. Diabetes Research and Clinical Practice, 91, 61-66. https://doi.org/10.1016/j.diabres.2010.09.024
[10] Bierhaus, A. and Nawroth, P.P. (2009) Multiple Levels of Regulation Determine the Role of the Receptor for AGE (RAGE) as Common Soil in Inflammation, Immune Responses and Diabetes Mellitus and Its Complications. Diabetologia, 52, 2251-2263. https://doi.org/10.1007/s00125-009-1458-9
[11] Bierhaus, A., Humpert, P.M., Morcos, M., Wendt, T., Chavakis, T., Arnold, B., Stern, D.M. and Nawroth, P.P. (2005) Understanding RAGE, the Receptor for Advanced Glycation End Products. Journal of Molecular Medicine, 83, 876-886. https://doi.org/10.1007/s00109-005-0688-7
[12] Schmidt, A.M., Yan, S.D., Yan, S.F. and Stern, D.M. (2000) The Biology of the Receptor for Advanced Glycation End Products and Its Ligands. Biochimica et Bio- physica Acta, 1498, 99-111. https://doi.org/10.1016/S0167-4889(00)00087-2
[13] Chavakis, T., Bierhaus, A. and Nawroth, P.P. (2004) RAGE (Receptor for Advanced Glycation End Products): A Central Player in the Inflammatory Response. Microbes and Infection, 6, 1219-1225. https://doi.org/10.1016/j.micinf.2004.08.004
[14] Ramasamy, R., Vannucci, S.J., Yan, S.S., Herold, K., Yan, S.F. and Schmidt, A.M. (2005) Advanced Glycation End Products and RAGE: A Common Thread in Aging, Diabetes, Neurodegeneration, and Inflammation. Glycobiology, 15, 16R-28R. https://doi.org/10.1093/glycob/cwi053
[15] Mallidis, C., Agbaje, I., Rogers, D., Glenn, J., McCullough, S., Atkinson, A.B., Steger, K., Stitt, A. and McClure, N. (2007) Distribution of the Receptor for Advanced Glycation End Products in the Human Male Reproductive Tract: Prevalence in Men with Diabetes Mellitus. Human Reproduction, 22, 2169-2177. https://doi.org/10.1093/humrep/dem156
[16] Karimi, J., Goodarzi, M.T., Tavilani, H., Khodadadi, I. and Amiri, I. (2011) Increased Receptor for Advanced Glycation End Products in Spermatozoa of Diabetic Men and Its Association with Sperm Nuclear DNA Fragmentation. Andrologia, 44, 280-286. https://doi.org/10.1111/j.1439-0272.2011.01178.x
[17] Saleh, R.A., Agarwal, A., Nelson, D.R., Nada, E.A., El-Tonsy, M.H., Alvarez, J.G., Thomas, A.J. and Sharma, R.K. (2002) Increased Sperm Nuclear DNA Damage in Normozoospermic Infertile Men: A Prospective Study. Fertility and Sterility, 78, 313-318. https://doi.org/10.1016/S0015-0282(02)03219-3
[18] Mahfouz, R., Sharma, R., Sharma, D., Sabanegh, E. and Agarwal, A. (2009) Diagnostic Value of the Total Antioxidant Capacity (TAC) in Human Seminal Plasma. Fertility and Sterility, 91, 805-811. https://doi.org/10.1016/j.fertnstert.2008.01.022
[19] Nakamura, H., Kimura, T., Nakajima, A., Shimoya, K., Takemura, M., Hashimoto, K.,et al. (2002) Detection of Oxidative Stress in Seminal Plasma and Fractionated Sperm from Subfertile Male Patients. European Journal of Obstetrics & Gynecology and Reproductive Biology, 105, 155-160. https://doi.org/10.1016/S0301-2115(02)00194-X
[20] Aitken, R.J. and Sawyer, D. (2003) The Human Spermatozoon—Not Waving but Drowning. In: Robaire, B. and Hales, B.F., Eds., Advances in Experimental Medicine and Biology, Springer, Boston, 85-98.
https://doi.org/10.1007/978-1-4419-9190-4_8
[21] Wautier, J.L. and Schmidt, A.M. (2004) Protein Glycation: A Firm Link to Endothelial Cell Dysfunction. Circulation Research, 95, 233-238. https://doi.org/10.1161/01.RES.0000137876.28454.64
[22] Chekir, C., Nakatsuka, M., Noguchi, S., Konishi, H., Kamada, Y., Sasaki, A., Hao, L. and Hiramatsu, Y. (2006) Accumulation of Advanced Glycation End Products in Women with Preeclampsia: Possible Involvement of Placental Oxidative and Nitrative Stress. Placenta, 27, 225-233. https://doi.org/10.1016/j.placenta.2005.02.016
[23] Roessner, C., Paasch, U., Kratzsch, J., Glander, H.J. and Grunewald, S. (2012) Sperm Apoptosis Signalling in Diabetic Men. Reproductive Biomedicine Online, 25, 292-299. https://doi.org/10.1016/j.rbmo.2012.06.004
[24] Yan, S.F., Barile, G.R., D’Agati, V., Du Yan, S., Ramasamy, R. and Schmidt, A.M. (2007) The Biology of RAGE and Its Ligands: Uncovering Mechanisms at the Heart of Diabetes and Its Complications. Current Diabetes Reports, 7, 146-153. https://doi.org/10.1007/s11892-007-0024-4
[25] Chohan, K.R., Griffin, J.T., Lafromboise, M., De Jonge, C.J. and Carrell, D.T. (2006) Comparison of Chromatin Assays for DNA Fragmentation Evaluation in Human Sperm. Journal of Andrology, 27, 53-59. https://doi.org/10.2164/jandrol.05068
[26] Evenson, D.P. and Wixon, R. (2006) Clinical Aspects of Sperm DNA Fragmentation Detection and Male Infertility. Theriogenology, 65, 979-991. https://doi.org/10.1016/j.theriogenology.2005.09.011
[27] Dominguez-Fandos, D., Camejo, M.I., Ballesca, J.L. and Oliva, R. (2007) Human Sperm DNA Fragmentation: Correlation of TUNEL Results as Assessed by Flow Cytometry and Optical Microscopy. Cytometry Part A, 71A, 1011-1018. https://doi.org/10.1002/cyto.a.20484
[28] Bungum, M., Humaidan, P., Spano, M., Jepson, K., Bungum, L. and Giwercman, A. (2004) The Predictive Value of Sperm Chromatin Structure Assay (SCSA) Parameters for the Outcome of Intrauterine Insemination, IVF and ICSI. Human Reproduction, 19, 1401-1408. https://doi.org/10.1093/humrep/deh280
[29] Sergerie, M., Laforest, G., Bujan, L., Bissonnette, F. and Bleau, G. (2005) Sperm DNA Fragmentation: Threshold Value in Male Fertility. Human Reproduction, 20, 3446-3451. https://doi.org/10.1093/humrep/dei231
[30] Baccetti, B., La Marca, A., Piomboni, P., Capitani, S., Bruni, E., Petraglia, F. and De Leo, V. (2002) Insulin-Dependent Diabetes in Men Is Associated with Hypothalamo-Pituitary Derangement and with Impairment in Semen Quality. Human Reproduction, 17, 2673-2677. https://doi.org/10.1093/humrep/17.10.2673
[31] Jutte, N.H., Grootegoed, J.A., Rommerts, F.F. and van der Molen, H.J. (1981) Exogenous Lactate Is Essential for Metabolic Activities in Isolated Rat Spermatocytes and Spermatids. Journal of Reproduction and Fertility, 62, 399-405. https://doi.org/10.1530/jrf.0.0620399
[32] Mita, M., Price, J.M. and Hall, P.F. (1982) Stimulation by Follicle-Stimulating Hormone of Synthesis of Lactate by Sertoli Cells from Rat Testis. Endocrinology, 110, 1535-1541. https://doi.org/10.1210/endo-110-5-1535
[33] Boussouar, F. and Benahmed, M. (2004) Lactate and Energy Metabolism in Male Germ Cells. Trends in Endocrinology & Metabolism, 15, 345-350. https://doi.org/10.1016/j.tem.2004.07.003
[34] Rato, L., Alves, M.G., Socorro, S., Duarte, A.I., Cavaco, J.E. and Oliveira, P.F. (2012) Metabolic Regulation is Important for Spermatogenesis. Nature Reviews Urology, 9, 330-228. https://doi.org/10.1038/nrurol.2012.77
[35] Alves, M.G., Socorro, S., Silva, J., Barros, A., Sousa, M., Cavaco, J.E. and Oliveira, P.F. (2012) In Vitro Cultured Human Sertoli Cells Secrete High Amounts of Acetate that Is Stimulated by 17β-Estradiol and Suppressed by Insulin Deprivation. Biochimica et Biophysica Acta, 1823, 1389-1394. https://doi.org/10.1016/j.bbamcr.2012.06.002
[36] Oliveira, P.F., Alves, M.G., Rato, L., Laurentino, S., Silva, J., Sá, R., Barros, A., Sousa, M., Carvalho, R.A., Cavaco, J.E. and Socorro, S. (2012) Effect of Insulin Deprivation on Metabolism and Metabolism-Associated Gene Transcript Levels of in Vitro Cultured Human Sertoli Cells. Biochimica et Biophysica Acta, 1820, 84-89. https://doi.org/10.1016/j.bbagen.2011.11.006
[37] Hall, P.F. and Mita, M. (1984) Influence of Follicle-Stimulating Hormone on Glucose Transport by Cultured Sertoli Cells. Biology of Reproduction, 31, 863-869. https://doi.org/10.1095/biolreprod31.5.863
[38] Angulo, C., Rauch, M.C., Droppelmann, A., Reyes, A.M., Slebe, J.C., Delgado-Lopez,F., Guaiquil, V.H. and Vera, J.C. (1998) Concha, II, Hexose Transporter Expression and Function in Mammalian Spermatozoa: Cellular Localization and Transport of Hexoses and Vitamin C. Journal of Cellular Biochemistry, 71, 189-203. https://doi.org/10.1002/(SICI)1097-4644(19981101)71:2%3C189::AID-JCB5%3E3.0. CO;2-R
[39] Seethalakshmi, L., Menon, M. and Diamond, D. (1987) The Effect of Streptozotocin-Induced Diabetes on the Neuroendocrine-Male Reproductive Tract Axis of the Adult Rat. The Journal of Urology, 138, 190-194. https://doi.org/10.1016/S0022-5347(17)43042-4
[40] Muralidhara, S.B. (2007) Early Oxidative Stress in Testis and Epididymal Sperm in Streptozotocin-Induced Diabetic Mice: Its Progression and Genotoxic Consequences. Reproductive Toxicology, 23, 578-587. https://doi.org/10.1016/j.reprotox.2007.02.001
[41] Cummins, J.M., Jequier, A.M. and Kan, R. (1994) Molecular Biology of Human Male Infertility: Links with Aging, Mitochondrial Genetics, and Oxidative Stress? Molecular Reproduction and Development, 37, 345-362. https://doi.org/10.1002/mrd.1080370314
[42] St John, J.C., Jokhi, R.P. and Barratt, C.L. (2005) The Impact of Mitochondrial Genetics on Male Infertility. International Journal of Andrology, 28, 65-73. https://doi.org/10.1111/j.1365-2605.2005.00515.x
[43] Lestienne, P., Reynier, P., Chretien, M.F., et al. (1997) Oligoasthenospermia Associated with Multiple Mitochondrial DNA Rearrangements. Molecular Human Reproduction, 3, 811-814. https://doi.org/10.1093/molehr/3.9.811
[44] Kao, S.H., Chao, H.T. and Wei, Y.H. (1998) Multiple Deletions of Mitochondrial DNA Are Associated with the Decline of Motility and Fertility of Human Spermatozoa. Molecular Human Reproduction, 4, 657-666. https://doi.org/10.1093/molehr/4.7.657
[45] Spiropoulos, J., Turnbull, D.M. and Chinnery, P.F. (2002) Can Mitochondrial DNA Mutations Cause Sperm Dysfunction? Molecular Human Reproduction, 8, 719-721. https://doi.org/10.1093/molehr/8.8.719
[46] Miller, D., Briggs, D., Snowden, H., Hamlington, J., Rollinson, S., Lilford, R., et al. (1999) A Complex Population of RNAs Exists in Human Ejaculate Spermatozoa: Implications for Understanding Molecular Aspects of Spermatogenesis. Gene, 237, 385-392. https://doi.org/10.1016/S0378-1119(99)00324-8
[47] Ostermeier, G.C., Dix, D.J., Miller, D., Khatri, P. and Krawetz, S.A. (2002) Spermatozoal RNA Profiles of Normal Fertile Men. The Lancet, 360, 772-777. https://doi.org/10.1016/S0140-6736(02)09899-9
[48] Ostermeier, G.C., Goodrich, R.J., Diamond, M.P., Dix, D.J. and Krawetz, S.A. (2005) Toward Using Stable Spermatozoal RNAs for Prognostic Assessment of Male Factor Fertility. Fertility and Sterility, 83, 1687-1694. https://doi.org/10.1016/j.fertnstert.2004.12.046
[49] Platts, A.E., Dix, D.J., Chemes, H.E., Thompson, K.E., Goodrich, R., Rockett, J.C., et al. (2007) Success and Failure in Human Spermatogenesis as Revealed by Teratozoospermic RNAs. Human Molecular Genetics, 16, 763-773. https://doi.org/10.1093/hmg/ddm012
[50] Van Houten, B., Woshner, V. and Santos, J.H. (2006) Role of Mitochondrial DNA in Toxic Responses to Oxidative Stress. DNA Repair, 5, 145-152. https://doi.org/10.1016/j.dnarep.2005.03.002
[51] Shoffner, J.M. and Wallace, D.C. (1994) Oxidative Phosphorylation Diseases and Mitochondrial DNA Mutations: Diagnosis and Treatment. Annual Review of Nutrition, 14, 535-568. https://doi.org/10.1146/annurev.nu.14.070194.002535
[52] Bennetts, L.E. and Aitken, R.J. (2005) A Comparative Study of Oxidative DNA Damage in Mammalian Spermatozoa. Molecular Reproduction and Development, 71, 77-87. https://doi.org/10.1002/mrd.20285
[53] Lewis, S.E., O’Connell, M., Stevenson, M., et al. (2004) An Algorithm to Predict Pregnancy in Assisted Reproduction. Human Reproduction, 19, 1385-1394. https://doi.org/10.1093/humrep/deh227
This article is excerpted from the Journal of Diabetes Mellitus by Wound World.


