Pressure ulcers (PU) are a crucial patient safety consideration in acute care settings (Lyder et al, 2008; National Institute for Health and Care Excellence, 2019). They are defined by the European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP), and Pan Pacific Pressure Injury Alliance (PPPIA) (EPUAP et al, 2019) as:
"Localised damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device. The injury can present as intact skin or an open ulcer and may be painful. The injury occurs as a result of intense and/or prolonged pressure or pressure in combination with shear. The tolerance of soft tissue for pressure and shear may also be affected by microclimate, nutrition, perfusion, comorbidities and condition of the soft tissue’."
It is well established that PUs are caused primarily by body weight pressure on the bony prominences. In contrast, MDRPU are caused when a device is in contact with the skin and the localised forces from the device deform the underlying skin and soft tissues (Gefen et al, 2020a; 2020b). MDRPU are sometimes termed device-related pressure ulcers (DRPU) to include devices that are not specifically medical such as mobile phones/objects left in the bed/chair (EPUAP et al, 2019). For the purpose of this article, we will discuss MDRPU as these are most commonly encountered in our clinical practice.
This article reports the techniques used to prevent MDRPU in a critical care setting. These techniques were commenced before the COVID-19 pandemic and were more important than ever during the COVID-19 pandemic, due to additional numbers of patients in the ICU with medical devices in place. Additionally, during the COVID-19 pandemic, an increased number of patients in the ICU were nursed prone (face down), adding additional pressure on the facial structure.
While not all PU are preventable, a range of measures were put in place to avoid those avoidable MDRPU in the ICU at the RUH. Critical care patients are at higher risk of all PU, particularly MDRPU due to the large numbers of devices in use in this patient population. These patients are more likely to experience lowered levels of consciousness, hallucinations and sensory perception inhibited, reduced mobility, vasopressor use, renal replacement therapy, malnutrition, hypovolemia, and hypotension. Because of this they are more reliant on medical devices.
It is necessary to consider what is meant by a MDRPU. The NPUAP (2016) suggests that MDRPU:
‘…result from the use of devices designed and applied for diagnostic or therapeutic purposes. The resultant pressure injury generally conforms to the pattern or shape of the device.
In contrast, a later definition was proposed by Gefen et al. (2020a) in an international consensus document that is more detailed:
‘A DRPU involves interaction with a device or object that is in direct or indirect contact with skin … or implanted under the skin, causing focal and localised forces that deform the superficial and deep underlying tissues. A DRPU, which is caused by a device or object, is distinct from a PU, which is caused primarily by body weight forces. The localised nature of device forces results in the appearance of skin and deeper tissue damage that mimics that of the device in shape and distribution.’
While there has been a reduction in the overall number of PU at the RUH over the past few years following a Rapid Spread programme (Heywood et al, 2018), the incidence of MDRPU has altered little. Figure 1 shows an example of MDRPU to the nose and the cheek.
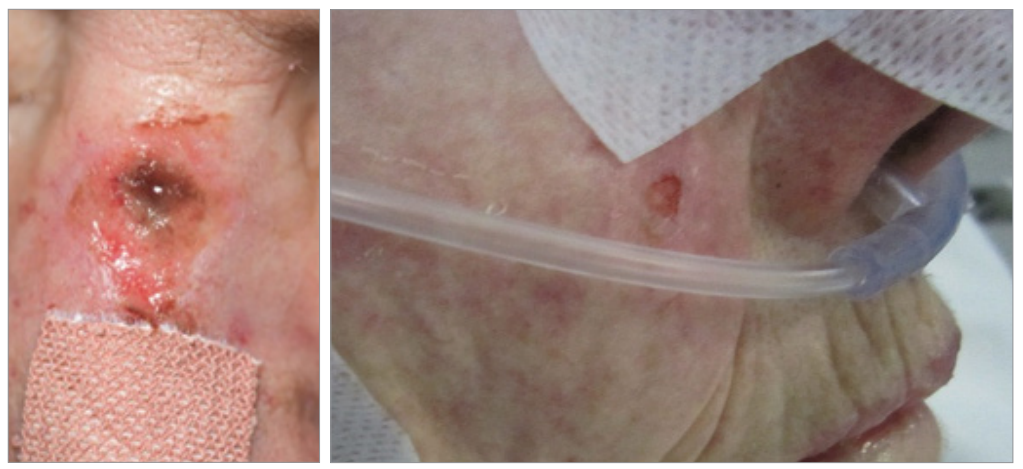
Figures 1. MDRPU to the nose and the cheek
Medical devices are mostly intended and applied for exploratory or therapeutic purposes. MDRPU in ICU affects patients of all ages and with all medical backgrounds. Understanding which devices cause MDRPU can assist with prevention strategies. EPUAP et al (2019) suggest a plethora of medical devices which may cause MDRPU, however this range is smaller in the ICU environment. Gefen et al (2020a) suggest that the types of devices that are most commonly cited as causing MDRPU are: endotracheal (ET) tubes and nasogastric (NG) tubes, oxygen tubing, non-invasive ventilation masks, urinary catheters, cervical collars and casts.
This contrasts with PU incidence data collected in ICU at the RUH, suggesting that MDRPU have occurred under ET tubes, nasal high flow, NG/ (nasojejunal) NJ tubes and urinary catheters. Table 1 outlines the differences between the MDRPU reported at the RUH and by Gefen et al (2020a). The RUH MDRPU incidence locations are in line with the findings of an earlier prospective study in 6 ICUs, which found that ET and NG tubes were the cause of most of the MDPU (Coyer et al, 2014). Although this study was based in the USA and Australia, it was a large study analysing 483 patients.
In a systemic review, Barakat-Johnson et al (2019) identified that a high proportion of patients in the ICU setting are at risk of not just MDRPU but all PU due to their limited mobility, often paired with multiorgan failure. Alongside this general elevated risk of PU, the high usage of medical devices in ICU also means that there is a high risk of MDRPU to ICU patients. MDRPU are frequently reported in intensive care and/or critical care settings due to the number of medical devices used in this inpatient population (Barakat-Johnson et al, 2017; 2019; Kayser et al, 2018; Garcia- Molina, 2018; Gefen et al, 2020a).
METHODS
Many patients nursed in the ICU at the RUH with the COVID-19 virus were proned, ventilated and sedated with very poor oxygenation and tissue perfusion for weeks. Ventilation and sedation, in order to take over the work of breathing for the patient in itself, means the patient relies solely on the nurse for any movement of the body. In addition, the sensory perception of pain or discomfort does not exist, or the ability to tell someone they are in pain. What also changed during the COVID-19 pandemic, was that the importance of multidisciplinary team (MDT) working was paramount to the success of all critical care environments. In ICU at the RUH, the development of a collaborative proning team ensured that pressure area care became the role of everyone, not just the bedside nurse.
The ICU at the RUH has a Tissue Viability Ambassador who is a Sister on the unit. She has pioneered practices to prevent PUs and developed robust pathways that are consistently implemented to avoid MDRPU. The role of the Tissue Viability Ambassador is valuable as this person becomes a subject expert in their own department. This is particularly important in the critical care setting, where nursing is different to anywhere else in a hospital. The ICU tissue viability ambassador is the person that others go to for resources and to troubleshoot issues in practice. This has been paramount to the reduction of MDRPU in ICU at the RUH.
The Tissue Viability Ambassador in ICU implemented many MRDPU prevention strategies, which are outlined in Table 2. These strategies, which have been consistently employed by the ICU team, have led to the low incidence of MDRPU in the RUH ICU.
Endotracheal tubes
ET tubes are an ICU device that carries a highrisk of MDRPU. Intubated patients require an ET tube being inserted, this flexible plastic tube is placed through the mouth into the trachea to help a patient breathe. The ET tube is then connected to a ventilator and the ET tube needs to be secured to the patient to avoid displacement. In most patients, an AnchorFast oral ET tube fastener is used to secure the ET tube (Figure 2). The AnchorFast has a one-click security clamp, which locks the ET tube in place. This is beneficial as one click easily releases the clamp for regular tube repositioning for PU prevention. The AnchorFast also has a nonabsorbent upper lip shaped pad, which helps keep the tube off the lip and skin barrier pads that fasten the device firmly in place. These medical devices such as ET, nasal high flow, non-invasive ventilation masks are rigid, tight and uncomfortable, but they are necessary lifesaving equipment. The devices need to be rigid to ensure that airways remain open for example, however this causes issues with the soft tissues when they are compressed beneath the medical device.
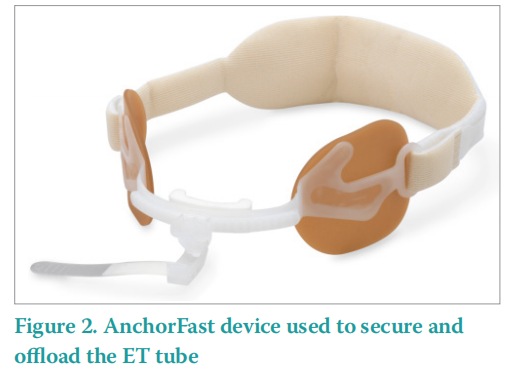
The AnchorFast oral ET tube fastener is not suitable for all patients. Those with facial oedema, facial hair and cranial or facial abnormalities may have to use an alternative device.
In this instance, a Velcro ET tube holder is used because of the soft materials, avoiding unnecessary pressure, while maintaining a secure fitting and a soft neck piece. The neckpiece is fitted with an adjustable laminated hook and loop fastening, which can be repositioned to size. The neckband should be as close to the corners of the patient’s mouth as possible for protection, to reduce pressure to the sides of the mouth. In addition, Kerrapro gel strips (Figure 3) are also used to further offload the pressure. The Kerrapro gel strips effectively redistribute this pressure, dispersing it over the pad to protect the skin.

NG and NJ tubes
NG and NJ tubes risk MDRPU to the septum. NG and/or NJ tubes are used in most intubated patients, as these patients will not be able to feed themselves. The main safety issue with these devices, is that they need to remain in place to avoid the risk of aspiration. They also need to be made of a rigid plastic that is firm enough to allow feed through the tubing, which is why they are a likely cause of a MDRPU. Bader et al (2019) outlined that these devices have been of generic design and made from stiff polymers, which can cause damage to the underlying soft tissues. Part of the reason that patients get MDRPU is because of the materials with which they are manufactured. These devices essentially have not changed much over time and likely not in the near future, but what we have changed is greater awareness of MDRPU within the critical care setting. This has helped with improving prevention practices.
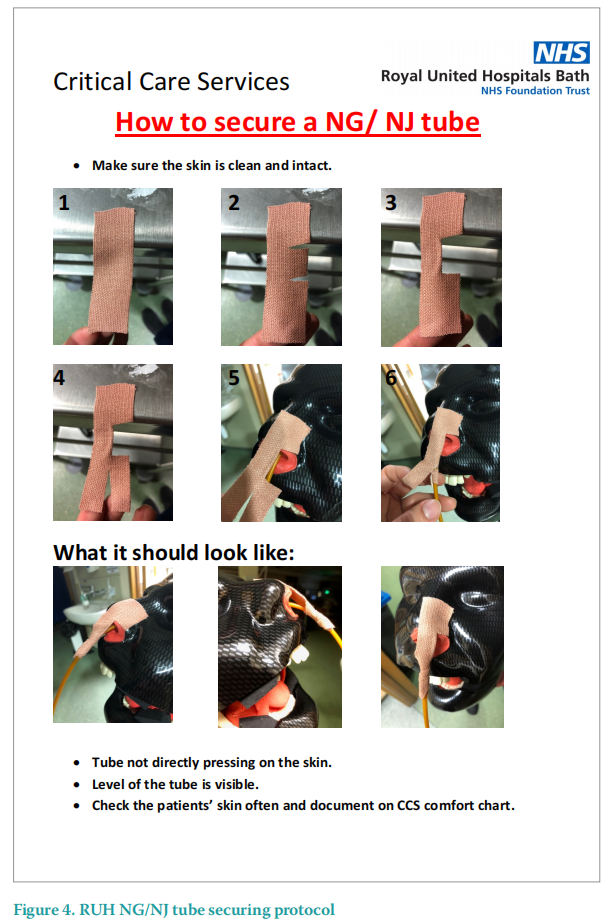
NG and NJ tubes are used frequently in the ICU in most intubated patients and other complex ICU patients. In order to avoid this type of MDRPU, a specific technique for securing the NG and NJ tubes has been developed and is outlined in Figure 4. This technique uses elastoplast to secure the tube and hold it away from the nose, reducing the risk of MDRPU. Additional barrier films can be applied before applying the Elastoplast and adhesive removers can be used to avoid trauma to the skin when removing the Elastoplast to renew it.
Nasal high flow
Patients who are not intubated but required a higher level of oxygen therapy were placed onto either Nasal High Flow (NHF; Figure 5) or non invasive ventilation (NIV). During the COVID-19 pandemic, increased amounts of NIV and NHF were used across the trust, due to increased oxygenation requirements, therefore the NIV or NHF supported respiratory function by forcing air into the lungs, lessening their respiratory effort.
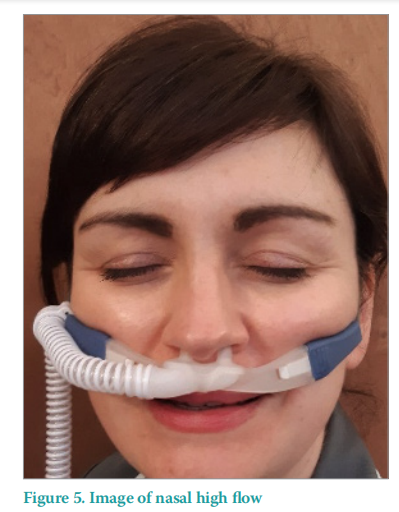
Both methods (NIV or NHF) use Kerrapro gel strips to offload pressure behind the ears to protect them from the straps. When using NHF the nostrils are at risk of MDRPU from the nasal prongs, which are made of firm material. The area of the nostrils under the on NHF has regular observation and position changes of the nasal prongs to avoid prolonged pressure. The NIV mask has Kerrapro gel strips placed under the mask on the bridge of the nose, as well as under the straps on the ears. The mask is regularly observation and the position of the mask on the bridge of the nose and around the ears is regularly changed.
These actions are performed as soon as the oxygen therapy has been initiated and they are prompted by the Critical Care comfort chart. This document has been designed to be specific to critical care and it prompts staff during pressure area care to observe and check all the medical devices the patient has and what action they have done. This means the patients skin checks with medical devices in places are checked 2–3 hourly.
Urinary catheters
Most patients in critical care have a urinary catheter in situ in order to accurately measure urine output, however there is a risk of MDRPU from the catheter tubing. Mostly, these are indwelling Foley catheters. Urine output is monitored hourly in the RUH ICU, meaning that every hour the catheter is checked and it would be quickly discovered if the catheter was potentially causing undue pressure. In ICU at the RUH urinary catheters are regularly inspected while assisting a patient with washing and hygiene needs. When repositioning a patient, the catheter is protected and moved so that it is not pressing against the skin causing undue pressure. There is a risk of a MDRPU from the catheter tubing if the patient is lying on it or if the device places pressure on the skin.
In addition, the Statlock device is used to secure the catheter tubing on each thigh, for those patients where it is useful. The Statlock is regularly repositioned when the patient is supported to move from side to side. Clinell continence care wipes are used for regular catheter entry points are checked.
The highest risk times are when a patient is lying on their side, and the inner thighs are at risk of damage from the catheter tubing. During the COVID-19 pandemic, patients were more frequently proned, or nursed on their fronts in order to maximise lung perfusion. The ICU proning protocol means that patients who require this intervention are proned for 18 hours, with only small positional changes of the head and neck. In this instance the catheter tubing is placed between the patients legs and is there for 18 hours with only minor positional changes. This obviously increases the risk of MDRPU from the urinary catheter. Alongside this risk, most critical care patients will be unable to vocalise any pain while they are intubated and sedated.
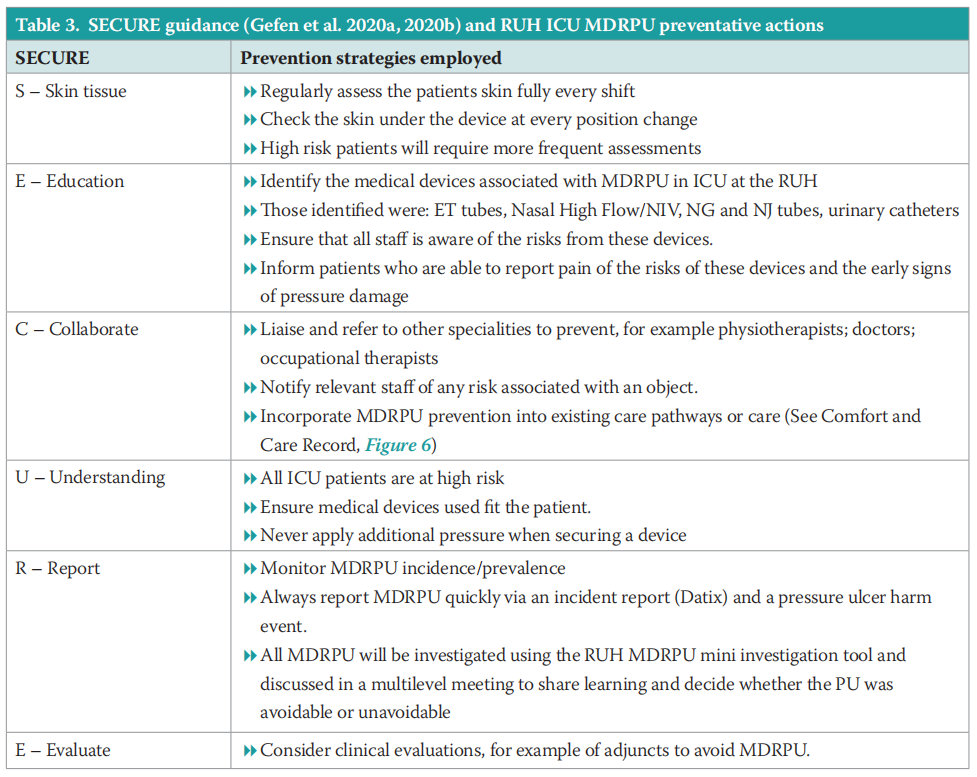
As well as the above methods that are specific to these four devices, the ICU team used the SECURE protocol to support MDRPU prevention. Gefen et al (2020a; 2020b) published a SECURE pathway to guide DRPU prevention. Nurses in the RUH ICU have adopted this pathway to support PU prevention. Table 3 outlines the actions that are taken in ICU at the RUH to prevent MDRPU.
The SSKIN bundle (NHS Midlands and East, 2012) has been used at the RUH to ensure that evidence-based, multifaceted PU prevention strategy is systematically embedded in practice. In the prevention of MDRPU, the most important elements of the SSKIN bundle are skin checking under medical devices and repositioning/keep moving (of medical devices). In ICU at the RUH, an ICU Comfort and Care Record (Figure 6) has been developed in order to ensure that the areas under these devices are regularly checked as well as the devices being regularly repositioned. This Comfort and Care Record ensures that the areas beneath endotracheal tubes, nasal high flow, face masks, NG/NJ tubes, catheters and other devices are checked daily. The Comfort and Care Record also ensures that additional adjuncts to the prevention of MDRPU such as Kerrapro gel strips are documented and evidenced.
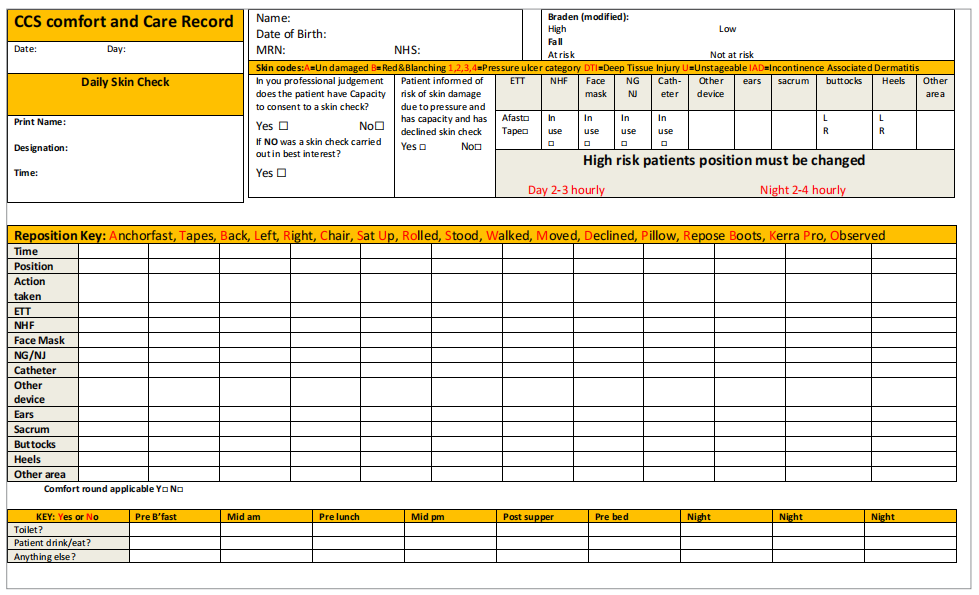
Figure 6. RUH ICU Comfort and Care Record
RESULTS
The Tissue Viability and ICU teams were expecting to see a rise in the number of MDRPUs and PUs overall during the COVID-19 pandemic. This was due to both an increased number of ICU admissions, as well as an increased use in medical devices such an NIV. Indeed, Vowden and Hill (2021) reported that the rate of PUs per 1000 beds increased from 1 pre pandemic to over 2.7 in the first month of the pandemic, in a large teaching hospital in the UK.
Figure 7 outlines the increase in 2020 and in particular in 2021 as a result of the COVID-19 pandemic. The virus started circulating in the UK in early 2020.
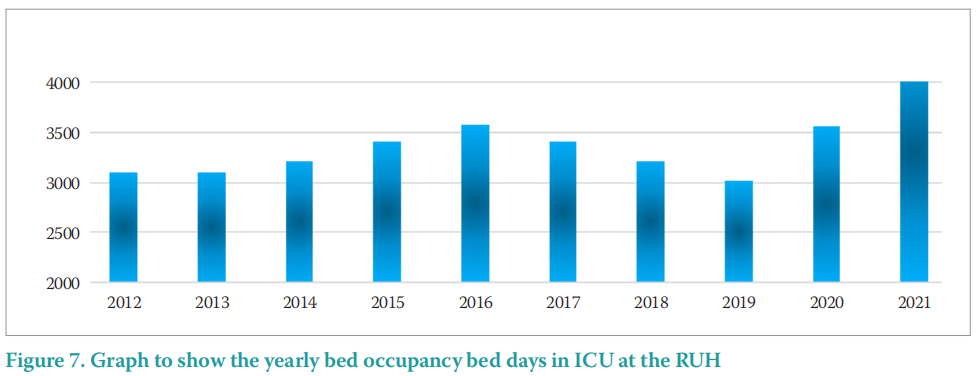
The MDRPU incidence outlined in Table 4 and Figure 8, demonstrates that there was no overall rise in the incidence of MDRPU over the COVID-19 pandemic period of 2019/20. Additionally, there was only a small rise (25%) of 1 additional PU in 2020/21.
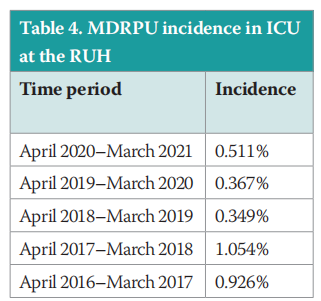
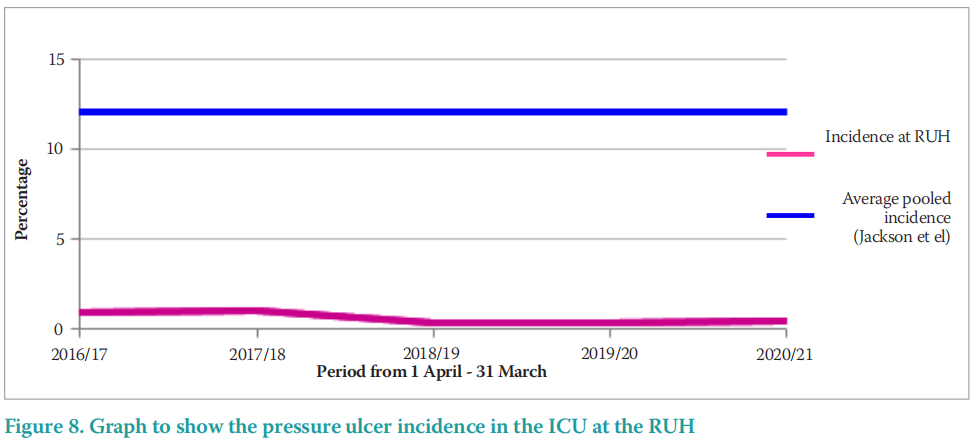
In comparison to a systematic review and metaanalysis, of 29 studies comprising 126,150 patients, Jackson et al (2019) identified an estimated pooled incidence of MDRPU of 12% (95% Confidence Interval [CI]: 8–18). The pooled incidence of 12% outlined by Jackson et al (2019; Figure 8) is far higher than the reported incidence in the RUH ICU that ranges from 0.367% to 1.054%. This may demonstrate the commitment to PU prevention of the ICU teams at the RUH.
The incidence of MDRPU in ICU at the RUH ranges from 0.349% to 1.054% and is far lower than that reported by Barakat-Johnson et al (2019). This may be in part due to the effective MDRPU prevention practices championed by the RUH ICU.
The MDRPU incidence outlined in Table 4 and Figure 8, demonstrates that there was no overall rise in the incidence of MDRPU over the COVID-19 pandemic period of 2019/20. Additionally, there was only a small rise (25%) of 1 additional PU in 2020/21.
In comparison to a systematic review and metaanalysis, of 29 studies comprising 126,150 patients, Jackson et al (2019) identified an estimated pooled incidence of MDRPU of 12% (95% CI: 8–18). The pooled incidence of 12% outlined by Jackson et al (2019; Figure 8) is far higher than the reported incidence in the RUH ICU that ranges from 0.367% to 1.054%. This may demonstrate the commitment to PU prevention of the ICU teams at the RUH.
Figure 9 outlines the numbers and sites of PU acquired on ICU at the RUH. These data report a sustained decrease in PU from the beginning of the project in 2016/17, until the data end point in 2021.
These data demonstrate a reduction from 12 PU in 2016/17 to 4 PU in 2020/21, a reduction of 66%. This is an achievement that has been celebrated. During the COVID-19 pandemic, there have been additional beds used in ICU at the RUH and it follows that PU were also expected to rise. Indeed, the RUH ICU is usually funded for 13 ICU beds and at the height of the COVID-19 pandemic saw 25 patients in ICU.
These results are of interest as a reduction in the MDRPU were expected as a result of this project, however, non-medical device related PU, such as those to heels and the sacrum, also decreased. There were no PU developed to the heel or sacrum after 2017/18. This could be as a result of an overall focus on PU reduction in ICU with an increased awareness of important facets such as skin checking. Aside from the sites expected in an acute setting of sacrum and heels, the majority of the PU reported were in areas in contact with medical devices (neck, lip, nostril, mouth, ear, nose).
Incidentally, 79% of the PU reported in ICU at the RUH from April 2016–March 2021 was on the head and neck. PU in the head and neck area can result in scarring, which can have a detrimental impact on body image and psychological health.
The MDRPU data presented (Figure 10) outlines an initial increase in MDRPU from 8 MDRPU in 2015/2016 to 9 MDRPU in 2017/2018. Despite this initial small rise, the subsequent data reported outlines a decrease in MDRPU that was sustained over the COVID-19 pandemic to 3 MDRPU in 2018/2019; 3 in 2019/2020 and 4 in 2020/2021.
Figure 9 outlines the numbers and sites of MDRPU acquired on ICU at the RUH. These data demonstrate a reduction from 8 MDRPU in 2016/17 to 4 MDRPU in 2020/2021. This represents another great achievement in a 50% reduction of MDRPU. There was an initial rise of 12.5% from 8 MDRPU in 2016/17 to 9 in 2017/18, but after that the reduction down to 3 or 4 PU annually has been sustained.
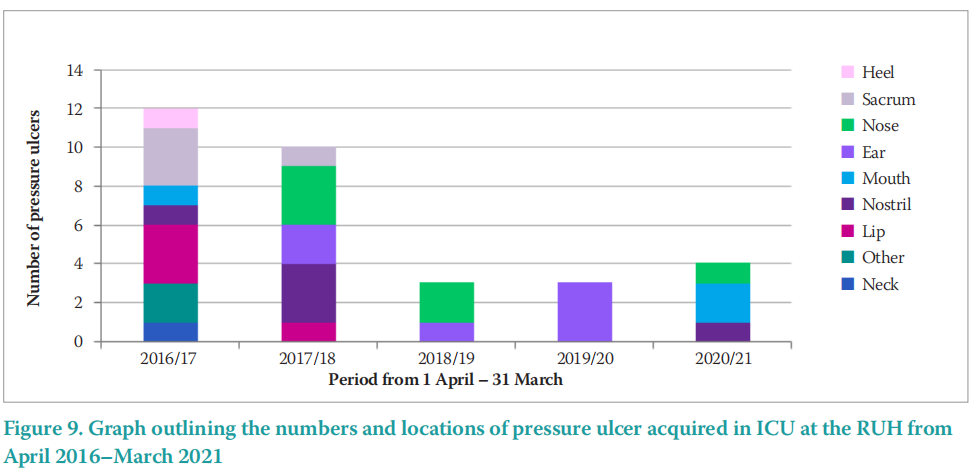
Along with an overall reduction in MDRPU, the locations of the PU are important to consider. Figure 10 outlines the locations of PU acquired in ICU at the RUH from 2016–2021. The highest incidence of PUs by location was the head and neck (58%), followed by mucosal membrane (21%), sacrum (14%), other (6%) and heel (1%). Of interest, the two most common sites for PU development (79%) were related to MDRPU — the head and neck and the mucosal membranes (lips and nose).
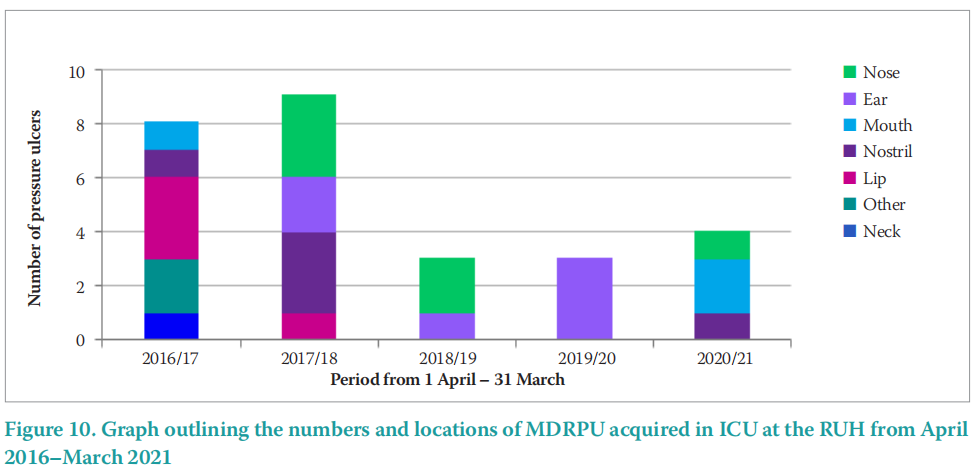
These reported data are in line with a recent systematic review of 13 studies, reported by BarakatJohnson et al (2017), which outlined the face, neck, ear and extremities as being the most common sites for PU development in critical care settings. It is important to understand the areas at the highest risk of PU development, in order that these particular areas can be closely monitored when skin checking and rotating medical devices.
It is well established that a proportion of PU acquired the in acute hospital setting are caused by medical devices. Between February 2019 and March 2021, 87% of PU acquired in the RUH ICU was MDRPU. This is greater than those reported by Black et al. (2010), who established that 34.5% of the PU in one US hospital was MDRPU. One reason for this difference is that the RUH ICU had already undertaken an extensive Rapid Spread Programme (Heywood et al, 2015; Heywood et al, 2018) to minimise all hospital acquired PU. However, this programme had only focussed on PU to common body sites such as the sacrum, buttocks and heels, not focussed on MDRPU.
CONCLUSION
MDRPU remain an issue in critical care settings due to the many medical devices used in this patient population. The RUH ICU has achieved a low MRDPU incidence due to the implementation of robust PU prevention pathways. Additionally, there has been more collaborative working of the whole MDT towards PU prevention. The role of the Tissue Viability Ambassador in ICU was key to delivering a low incidence of MDRPU. Actions were taken in ICU at the RUH to reduce pressure from MDRPU under key devices including: ET tubes, NG and NJ tubes, nasal high flow and urinary catheters. These actions include regular skin checking beneath the devices, regular rotation of the devices and the use of adjuncts such as the Anchorfast device for ET tubes and the Statlock device for urinary catheters. The SECURE pathway (Gefen et al 2020) was used to support the delivery of PU reduction strategies. The ICU comfort and care record assisted with the documentation of regular skin checking and offloading of pressure sites under medical devices. In a time when critical care saw very large volumes of sick, poorly perfused, hypoxic patients, the low incidence of MDRPU reflects how effective these prevention strategies have been.
REFERENCES
1. Bader D L, Worsley P R, Gefen A (2019) Bioengineering considerations in the prevention of medical device related pressure ulcers. Clin Biomech (Bristol, Avon). 67:70–7. https://doi.org/10.1016/j. clinbiomech.2019.04.018
2. Barakat-Johnson M, Barnett CW, Wand T, White K (2017) Medical device-related pressure injuries: An exploratory descriptive study in an acute tertiary hospital in Australia. J Tissue Viability 26(4):246–53. https://doi.org/10.1016/j.jtv.2017.09.008
3. Barakat-Johnson M, Lai M, Wand T et al (2019) The incidence and prevalence of medical device-related pressure ulcers in intensive care: systematic review. J Wound Care 28(8):512–21. https://doi. org/10.12968/jowc.2019.28.8.512
4. Black JM, Cuddigan JE, Walko MA et al (2010) Medical device related pressure ulcers in hospitalized patients. Int Wound J 7(5):358–65. https://doi.org/10.1111/j.1742-481x.2010.00699.x
5. Coyer FM, Stotts NA, Blackman VS (2014) A prospective window into medical device-related pressure ulcers in intensive care. Int Wound J 11(6);656–64. https://doi.org/10.1111/iwj.12026
6. European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP), Pan Pacific Pressure Injury Alliance (PPPIA) (2019) Prevention and Treatment of Pressure Ulcers/ Injuries: Clinical Practice Guideline. The International Guideline. Haesler E (ed)
7. García-Molina P, Balaguer-López E, García-Fernández FP et al. (2018) Pressure ulcers’ incidence, preventive measures, and risk factors in neonatal intensive care and intermediate care units. Int Wound J 15(4):571–9. https://doi.org/10.1111/iwj.12900
8. Gefen A, Alves P, Ciprandi G. et al (2020) An international consensus on device related pressure ulcers: SECURE prevention. Br J Nurs 29(5):S36–7. https://doi.org/10.12968/bjon.2020.29.5.s36
9. Gefen, A, Alves, P Ciprandi et al. (2020) Device related pressure ulcers: SECURE prevention. J Wound Care 29(Sup2a):S1–52. https://doi. org/10.12968/jowc.2020.29.sup2a.s1
10. Heywood N, Brown L, Arrowsmith M, and Poppleston A (2018) After Rapid Spread, sustaining a low pressure ulcer incidence in an acute Trust. Tissue Viability Society 2018
11. Heywood N, Arrowsmith M, Poppleston A (2015) Using Rapid Spread methodology to reduce the incidence of hospital-acquired pressure ulcers. Wounds UK 11(12):42–50. https://tinyurl.com/2p8685fk (accessed 5 June 2022)
12. Jackson, D Sarki A M, Betteridge R Brooke D (2019) Medical devicerelated pressure ulcers : A systematic review and mete analysis. Int J Nurs Stud 92 :109–20. https://doi.org/10.1016/j.ijnurstu.2019.02.006
13. Kayser SA VanGilder CA Ayello EA, Lachenbruch C (2018) Prevalence and analysis of medical device related pressure injuries: results from the international pressure ulcer prevalence survey. Adv Skin Wound Care 31(6):276–85. https://doi.org/10.1097/01. asw.0000532475.11971.aa
14. Lyder CH, Ayello EA (2008) Pressure ulcers: a patient safety issue. In: Hughes RG (ed) Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008
15. Moore Z, McEvoy N, Asvar P et al (2021) Facial pressure injuries and the COVID-19 pandemic. J Wound Care 30 (3):162–9. https://doi. org/10.12968/jowc.2021.30.3.162
16. National Institute for Health and Care Excellence (2014) CG 179 Pressure ulcer prevention and management (Online). https://www. nice.org.uk/guidance/cg179 (accessed 5 June 2022)
17. NPIAP (2020) Best practices for the prevention of medical device related pressure injuries in Critical Care (Online). Available from: BestPracticesCriticalCare-2020 (ymaws.com) (Accessed 7 October 2021)
18. National Pressure Ulcer Advisory Panel (NPUAP), (2016). Pressure Injury stages. https://tinyurl.com/tu3kjwh (accessed 5 June 2022)
19. NHS Midlands and East (2012) Five simple steps to prevent and treat pressure ulcers. (Online). http://nhs.stopthepressure.co.uk/ (accessed 5 June 2022)
20. Vowden K, Hill L (2021) What is the impact of COVID-19 on tissue viability services and pressure ulceration? J Wound Care 30(7):522–31. https://doi.org/10.12968/jowc.2021.30.7.522
This article is excerpted from the Wounds UK | Vol 18 | No 2 | 2022 by Wound World.


