1. Introduction
Since ancient times, many plants have been used to treat many diseases. Medicinal plants and microorganisms were the major sources of medicine over many centuries [1]. While a majority of new drugs have been generated from natural products and their derivatives [2] [3].
One of the most important genera of Alliaceae family is Allium species and also among the oldest cultivated vegetables which have been used as ornamentals, spices, vegetables, or as medicines for curing various diseases. This activity is mainly attributed to the presence of the sulfur compounds in their compositions [4] [5]. Most common species in this genus include: Allium sativum (Garlic), Allium cepa (Onion), Allium ascalonicum (Shallot), Allium porrum (Leek), Allium toberosum (Chinese Chive), Allium schoenoprasum (Chive) Allium sativum (Garlic) and Allium cepa (Onion) [4].
Leek (Allium porrum) is one of the allium families consumed in all continents as food and traditional medicine. It contains bioactive components with health promoting properties such as antimicrobial, antitumorigenetic, immunomodulatory properties and more [6]. Allium sativum (garlic) is known to contain a variety of biologically active chemical constituents, such as alliin, allicin, alliinase, S-allylcysteine and other organo-sulfur compounds. The antimicrobial properties of garlic have been attributed to the presence of alliin and allicin. This is suggestive that garlic could be active against bacteria associated with wound infection [7] [8].
Chronic wound management is a burden to patients all over the world, especially in poor countries with inadequate health care facility [9]. An infected wound activates leukocytes which in turn release cytolytic enzymes. This induces an imbalance between pathological local factors and integrity of immune defenses. This promotes colonization of both Gram-positive and Gram-negative bacteria such as, particularly Staphylococcus, Enterococcus, Enterobacter, Pseudomonas and Finegoldia [10] [11] [12].
This study is designed to determine the antibacterial effect of garlic (Allium sativum) and leek (Allium porrum) on wound isolates assessing and to determine their synergistic effects.
2. Materials and Methods
2.1. Ethical Approval
The proposal for this study was submitted to the ethical committee for approval at Chukwuemeka Odumegwu Ojukwu University Teaching Hospital, Amaku, Anambra State, Nigeria. After due consideration, the approval was issued.
2.2. Study Period
This study was carried out over a period of six (6) months; December 2021 to May 2022.
2.3. Wound Sample Size
The sample size was decided based on the availability of patients with clinical evidence of wound infections at the time of sample collection.
2.4. Collection of Plant Samples
Garlic and leek were collected from the local market at Awkuzu, in Anambra state, Nigeria. The bulbs were identified and authenticated at the Department of Agricultural Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam, Anambra State, Nigeria.
2.5. Ethanolic Extraction Method
Following the method by [13], fresh garlic (Allium sativum) and leek (Allium porrum) bulbs were purchased from local market. Two kilograms (2.0 kg) each of garlic and leek bulbs were purchased. The garlic and leek bulbs were washed thoroughly under tap running water, aseptically cut into small pieces with a knife and then kept in the shade for 7 days at 32˚C - 35˚C. The semi-dried pieces were then crushed using pestle and mortar, and left to dry in the shade at room temperature for further 7 days. The dried garlic materials were further ground to powdery form with an electric blender. Two hundred gram (200 g) of garlic powder was extracted with 500 ml of ethanol for 24 hours by using Soxhlet apparatus. The extracts were concentrated using a rotary evaporator at 40˚C.
2.6. Aqueous Extraction Method
The dry peel around the bulbs of Garlic and Leek were removed and they were used to prepare extract. Both aqueous extracts were prepared using 100 g of fresh rootless bulbs and 100 ml of distilled/deionized water. Both plants were ground in a blender for 10 minutes and filtered using qualitative paper filters, and sterilized through 0.2um membrane filters using a vacuum pump. All residues were weighed, and the concentration of the final solution of both extracts was considered 25% (w/v) or 250 mg/ml, as previously described [8].
2.7. Collection of Wound Swabs
The wound swabs were obtained after cleaning the wound surfaces with saline from patients with clinical evidence of wound infection at Chukwuemeka Odumegwu Ojukwu University Teaching Hospital Amakwu and Nnamdi Azikiwe University Teaching Hospital Nnewi, both in Anambra state, Nigeria. Each swab was placed in separate tubes containing transport medium and sent to the Microbiology laboratory for further culture analysis. Ten samples were collected from ten different patients when they visited the hospital [14].
2.8. Microbiological Analysis
The swabs were streaked on blood agar and MacConkey agar and incubated at 35˚C - 37˚C for 24 - 48 hours. Bacteria colonies were identified by morphologic characteristics such as haemolysis on blood agar, changes in physical appearance in differential media and enzyme activities of the organisms. Gram reaction and other biochemical tests namely: Indole, Citrate, Catalase, coagulase and oxidase tests were also done for identification of the isolates using methods described by [15].
2.9. Antibacterial Susceptibility Testing
Susceptibility testing was performed by agar well diffusion technique. The inocula were suspended in sterile normal saline and the density of suspension was determined by comparison with McFarland 0.5 Barium sulphate solution. The test organism was uniformly seeded over the Mueller-Hinton agar surface. The plates were allowed to dry for 15 minutes and wells with a 6 mm diameter were punched out using a cork borer. The extracts from garlic and leek were used to fill the wells respectively at a concentration of 250 mg/ml and 500 mg/ml respectively. Gentamicin and Tetracycline were used as positive controls. The plates were incubated for 24 hours and inhibition zone diameters were measured to the nearest millimeter.
2.10. Statistical Analysis
The statistical analytical method employed in this study was Bonferroni test used for statistical analysis (P ≤ 0.05) to show if there are any significant differences among all studies obtained (Hamza, 2015).
3. Results
3.1. Bacterial Profile
A total of (10) bacteria isolates were obtained, four (4) were Gram-positive and six (6) were Gram-negative bacteria: three (3) Klesiella pneumoniae, two (2) Staphylococcus aureus, two (2) Enterococcus spp, two (2) Pseudomonas aeruginosa and one (1) E. coli. Table 1 shows the morphological characteristics of the organisms on blood and Mac Conkey agar. It also highlights the results of the Gram staining reaction and other biochemical tests carried on each of the isolates.
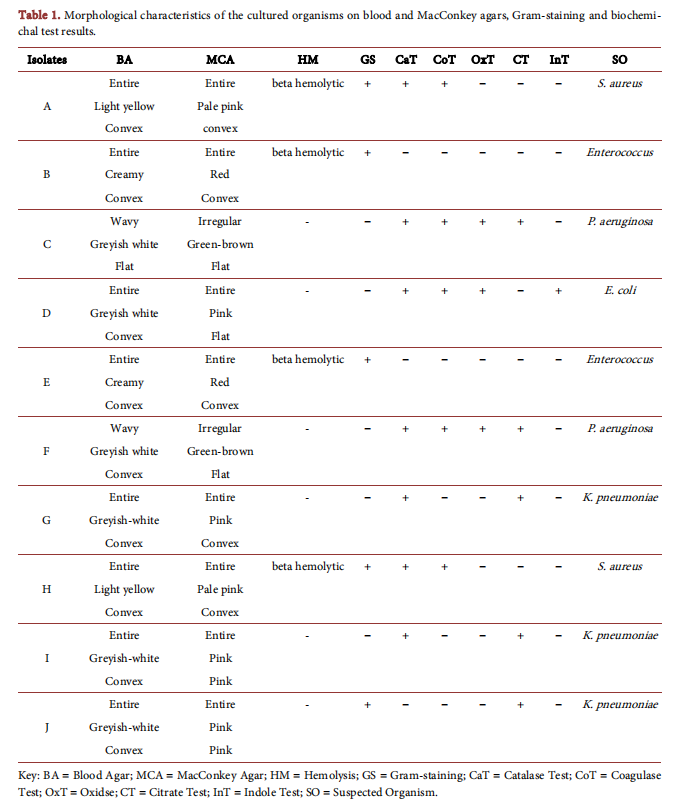
3.2. Antibacterial Susceptibility Testing
Both the aqueous and ethanolic extracts of garlic and leek were tested against the isolated and identified bacteria strains, with Gentamicin and Tetracycline as positive controls. Freshly prepared 500 mg/ml and 250 mg/ml of the ethanolic extracts of Garlic and Leek, and 250 mg/ml of aqueous extracts of both plants were tested against each of the 10 selected bacteria isolates. A mixture of 500 mg/ml each of the ethanolic extracts of both plants was also tested against each of the isolated organisms. The decision to use 250 mg/ml and 500 mg/ml concentrations was informed by a study where concentrations below 25% (250 mg/ml) of leek (Allium porrum) produced no effect against tested organisms [16] obtained are as follows:
Figure 1 shows the antimicrobial inhibition zones of aqueous garlic and leek and conventional antibiotics (gentamicin and tetracycline) on the bacterial species. All the bacteria isolates were resistant to aqueous garlic extract except one strain of P. aeruginosa which produced 20 mm zone of inhibition. Aqueous extract of leek produced zones of inhibition against both strains of Enterocuccus (19 mm and 17 mm respectively), all the strains of K. pneumoniae (16 mm, 16mm and 20 mm respectively), one strain of P. aeruginosa (20 mm) and one strain of S. aureus (19 mm).
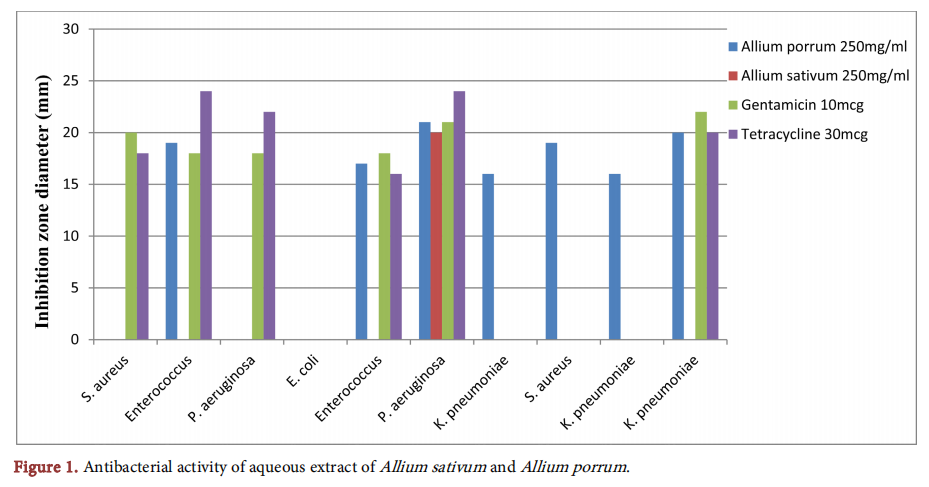
Figure 2 shows the antibacterial inhibition zones of ethanolic extracts of garlic and leek. One strain of P. aeruginosa was observed to be resistant to both ethanolic extracts of garlic and leek but susceptible to gentamicin and tetracycline with 18 mm and 22 mm zones of inhibition respectively. Two (2) strains of K. pneumoniae and one strain of S. aureus which were resistant to gentamicin and tetracycline were susceptible to the ethanolic extracts of both garlic and leek at both concentrations used producing varying degrees of zones of inhibition. The maximum inhibition zone to garlic ethanolic extract (500 mg/ml) was observed against one strain of P. aeruginosa (30 mm) and that of leek ethanolic extract (500 mg/ml) was observed against same organism (22 mm).
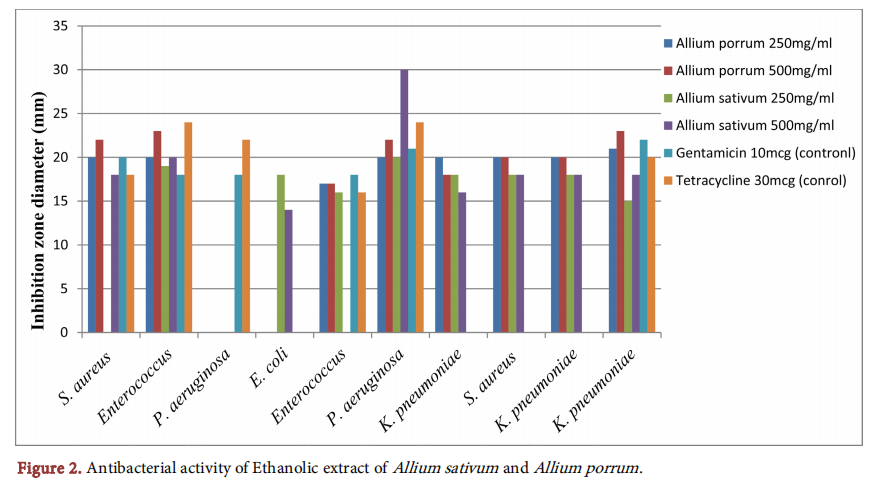
Figure 3 shows the antibacterial inhibition zones of combined ethanolic extracts of garlic and leek (500 mg/ml each). The combination of both ethanolic extracts produced no effect against one of the strains of P. aeruginosa just like the individual extracts. It produced no synergistic or additive effects on any of the organisms tested, only antagonistic and indifferent effects.
4. Discussion
In management of wound infections, antibiotic treatment is recommended and paramount, but then, an antibiotic susceptibility test should be performed [14]. The incidence of wound infection is more common in males than in females as 70% of the wound swabs collected were from male patients in the two facilities under study. These statistics agree with a study done in Ethiopia where the incidence of wound infection was more common in males (89.7%) than in females (81.4%) [15]; and in Nigeria where a study showed that the incidence of wound infection is higher in males (59.3%) than in females (40.7%) [17]. This might be explained by the fact that traditionally, in these countries, men are involved in occupations that may expose them to occupational hazards.
The organisms identified namely Klebsiella pneumoniae (30%), Staphylococcus aureus (20%), Pseudomonas aeruginosa (20%), Enterococcus (20%) and E. coli (10%) are similar to the findings of Mama et al., (2014) where S. aureus and E. coli were the most isolates from wounds. The high prevalence of S. aureus therefore is not unexpected since the organism is a normal flora on the skin. Since K. pneumoniae are typically nosocomial infections, patients may have contracted the infection through contaminated hands of medical personnel or through contaminated medical equipments and from the hospital environments [18]. The high percentage of mono microbial growth reported in this study which may be due to pre treatment by the patients before hospital visits were also confirmed by [19] [20] with 86% - 100% mono prevalence rate.
It was observed in this study that the ethanolic extracts (250 mg/ml and 500 mg/ml) of both plants acted as better antibacterial agents than the aqueous extracts. The aqueous extract of garlic produced antibacterial effect on P. aeruginosa strain, while the ethanolic extract had effect on all the isolates except a strain of P. aeruginosa in both concentrations. Similar studies by Akrayi [16] and Evans & Cowan [21] done with ethanolic exracts of Allium porrum and Lepidium sativum produced better inhibition zones than their aqueous extracts of the same concentration. The P. Aeruginosa isolate which was resistant to both plant extracts was susceptible to the two conventional antibiotics, Gentamicin and Tetracycline used as positive controls. It was also observed that there were wider inhibition zone diameters with 500 mg/ml when compared to half the concentration. This was also observed by Akrayi [16] in their study where slight increase in zones of inhibition diameter was observed with the ethanolic leek extract.
Combination of both plant extracts did not produce any synergistic effect as no appreciable increases in inhibition zone diameters were observed. This suggests that the extracts produce better activities against the isolated organisms when used separately than in combination, though this may require further investigation. The antibacterial activity of crude extracts of garlic and leek were also compared with the conventional antibiotics used as positive controls in this study, as was also observed in a study by Naem & Hadi [22].
The findings in this study with extracts of Allium sativum and Allium porrum confirm their antibacterial activities against both Gram-positive and Gram-negative organisms and may be considered as alternative treatment especially with continuous rise in antibiotic resistance.
5. Limitation of the Study
The authors of this study did not determine and isolate the active phytochemical constituents of these plant extracts responsible for antibacterial activity.
6. Conclusion
The applications of herbal products for the control of infections are emerging alternatives due to rapid development of resistance against antibiotics. This study underscores the potentials of Allium sativum and Allium porrum in the treatment of wound infections. Further studies on dosage, purification and identification of phytochemical components with antibacterial effect are advised.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
[1] Calixto, J.B. (2019) The Role of Natural Products in Modern Drug Discovery. Anais Da Academia Brasileira de Ciencias, 91, 1-7. https://doi.org/10.1590/0001-3765201920190105
[2] Katz, L. and Baltz, R.H. (2016) Natural Product Discovery: Past, Present, and Future. Journal of Industrial Microbiology and Biotechnology, 43, 155-176. https://doi.org/10.1007/s10295-015-1723-5
[3] Li, J.W. and Vederas, J.C. (2009) Drug Discovery and Natural Products. Science, 325, 161-165. https://doi.org/10.1126/science.1168243
[4] Mnayer, D., Fabiano-Tixier, A.S., Petitcolas, E., Hamieh, T., Nehme, N., Ferrant, C., Fernandez, X. and Chemat, F. (2014) Chemical Composition, Antibacterial and Antioxidant Activities of Six Essentials Oils from the Alliaceae Family. Molecules, 19, 20034-20053. https://doi.org/10.3390/molecules191220034
[5] Beshbishy, A., Wasef, L., Elewa, Y., Al-Sagan, A., Abd El-Hack, M., Taha, A. and Abd-Elhakim, Y. (2020) Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum): A Review. Nutrients, 12, 872. http://search.proquest.com/docview/2420177570 https://doi.org/10.3390/nu12030872
[6] Radovanović, B., Mladenović, J., Radovanović, A., Pavlović, R. and Nikoli, V. (2015) Phenolic Composition, Antioxidant, Antimicrobial and Cytotoxic Activites of Allium porrum L. (Serbia) Extracts. Journal of Food and Nutrition Research, 3, 564-569.
[7] Alhashim, M. and Lombardo, J. (2018) Mechanism of Action of Topical Garlic on Wound Healing. Dermatologic Surgery: Official Publication for American Society for Dermatologic Surgery, 44, 630-634. https://doi.org/10.1097/DSS.0000000000001382
[8] Venâncio, P.C., Figueroba, S.R., Nani, B.D., Nunes Ferreira, L.E., Muniz, B.V., Del Fiol, F.S., Sartoratto, A., Ribeiro Rosa, E.A. and Groppo, F.C. (2017) Antimicrobial Activity of Two Garlic Species (Allium sativum and A. tuberosum) against Staphylococci Infection in Vivo Study in Rats. Advanced Pharmaceutical Bulletin, 7, 115-121. https://doi.org/10.15171/apb.2017.015
[9] Ait Abderrahim, L., Taïbi, K., Ait Abderrahim, N., Boussaid, M., Rios-Navarro, C. and Ruiz-Saurí, A. (2019) Euphorbia Honey and Garlic: Biological Activity and Burn Wound Recovery. Burns, 45, 1695-1706. https://doi.org/10.1016/j.burns.2019.05.002
[10] Mikaili, P., Maadirad, S., Moloudizargari, M. and Aghajanshakeri, S. (2013) Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iranian Journal of Basic Medical Sciences, 16, 1031-1048.
[11] Roy, S., Santra, S., Das, A., Dixith, S., Sinha, M., Ghatak, S., Ghosh, N., Khanna, S., Mathew-steiner, S., Das, P., Blackstone, B.N., Powell, H.M., Valerie, K., Wozniak, D.J. and Sen, C.K. (2021) Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Annals of Surgery, 271, 1174-1185. https://doi.org/10.1097/SLA.0000000000003053
[12] Serra, R., Grande, R., Butrico, L., Rossi, A., Settimio, U.F., Caroleo, B., Amato, B., Gallelli, L. and De Franciscis, S. (2015) Chronic Wound Infections: The Role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Review of Anti-Infective Therapy, 13, 605-613. https://doi.org/10.1586/14787210.2015.1023291
[13] Abubakar, E.M.M. (2009) Efficacy of Crude Extracts of Garlic (Allium sativum) against Nosocomial Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae and Pseudomonas aeruginosa. Journal of Medicinal Plants Research, 3, 179-185.
[14] Bessa, L.J., Fazii, P., Di Giulio, M. and Cellini, L. (2015) Bacterial Isolates from Infected Wounds and Their Antibiotic Susceptibility Pattern: Some Remarks about Wound Infection. International Wound Journal, 12, 47-52. https://doi.org/10.1111/iwj.12049
[15] Mama, M., Abdissa, A. and Sewunet, T. (2014) Antimicrobial Susceptibility Pattern of Bacterial Isolates from Wound Infection and Their Sensitivity to Alternative Topical Agents at Jimma University Specialized Hospital, South-West Ethiopia. Annals of Clinical Microbiology and Antimicrobials, 13, Article No. 14. https://doi.org/10.1186/1476-0711-13-14
[16] Akrayi, H.T.J. (2012) Antibacterial Activity of Lepidium sativum and Allium porrum Extracts and Juices against Some Gram Positive and Gram Negative Bacteria. Medical Journal of Islamic World Academy of Sciences, 20, 10-16.
[17] Ohalete, C., Obi, R. and EmeaKoroha, M. (2012) Bacteriology of Different Wound Infection and Their Antimicrobial Susceptibility Patterns in IMO State Nigeria. World Journal of Pharmacy and Pharmaceutical Sciences, 1, 1155-1172.
[18] Gelaw, A., Gebre-Selassie, S. and Tiruneh, M. (2014) Isolation of Bacterial Pathogens from Patients with Postoperative Surgical Site Infections and Possible Sources of Infections at the University of Gondar Hospital, Northwest Ethiopia. Journal of Environmental and Occupational Health, 3, 103-108. https://doi.org/10.5455/jeos.20140512124135
[19] Bibi, S., Channa, G.A., Siddiqui, T.R. and Ahmed, W. (2012) Pattern of Bacterial Pathogens in Postoperative Wounds and Their Sensitivity Patterns. Journal of Surgery Pakistan (International), 17, 164-167.
[20] Sanjay, K.R., Prasad, M.N.N. and Vijaykumar, G.S. (2010) A Study on Isolation and Detection of Drug Resistance Gram Negative Bacilli with Special Importance to Post Operative Wound Infection. Journal of Microbiology and Antimicrobials, 2, 68-75.
[21] Evans, S.M. and Cowan, M.M. (2016) Plant Products as Antimicrobial Agents. Cosmetic and Drug Microbiology, 12, 205-231.
[22] Naem, R.K. and Hadi, N.A. (2012) The Antimicrobial Activity of Allium porrum Water Extract against Some Pathogenic Bacteria. Journal of Kerbala University, 10, 45-49.
This article is excerpted from the Open Journal of Internal Medicine by Wound World.


