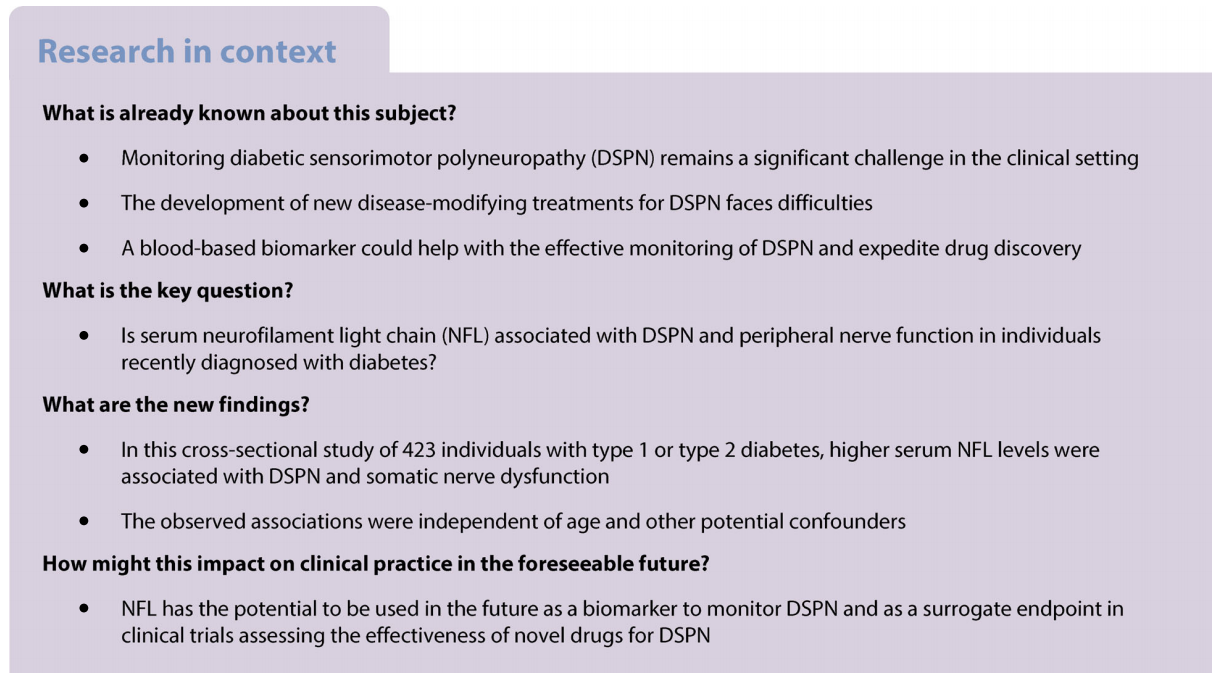
Introduction
Diabetic sensorimotor polyneuropathy (DSPN) affects individuals with diabetes or impaired glucose tolerance and/or impaired fasting glucose and is characterised by demyelination and axonal loss of peripheral sensory and motor nerves [1]. Despite the significant advances in elucidating the underlying pathogenesis of DSPN, the condition remains underdiagnosed and undertreated in clinical practice and poses major challenges in clinical trials [2, 3]. On the one hand, a delayed diagnosis may foster irreversible neuropathic damage, hamper suitable interventions and increase the risk of associated disabilities and medical costs. On the other hand, the early detection of subclinical or asymptomatic DSPN with electrophysiological or quantitative sensory testing, which could allow for early intervention [4], is time-consuming, expensive, requires expertise and is only accessible in specialised centres. Thus, there is an increased need for simple, easy-to-perform, inexpensive biomarker measurements that can provide clinical information that reasonably reflect early DSPN detected and monitored by peripheral nerve function tests. Beyond difficulties in diagnosis and management, developing new disease-modifying treatments for DSPN is an unmet need, as underlined by numerous failed phase 3 trials [5]. A fundamental reason for these unsuccessful trials is the lack of reproducible and accurate surrogate endpoints predicting the ultimate clinical endpoints such as foot ulceration and amputation [2]. Clinical symptoms and neurological deficits are subjective and, consequently, they have poor reproducibility [6], while electrophysiological studies represent US Food and Drug Administration (FDA)-approved objective surrogate endpoints [7], albeit their reproducibility has been questioned because of high inter-observer variability [6].
Therefore, there is a pressing demand to identify and validate novel biomarkers to monitor DSPN and facilitate drug discovery. Ideally, these biomarkers should be blood-based and easy to measure to expedite their implementation in the clinical setting. Additionally, their measurement should be objective, reproducible and accurate to allow their use as biomarkers for the progression of DSPN in clinical trials.
The neurofilament light chain (NFL) is a cytoskeletal component of mature neurons that provides structural stability and determines axonal diameter [8, 9]. NFL is more abundant in large myelinated axons facilitating a faster conduction velocity. Upon axonal injury, NFL is released from axons into the circulation [10]. The efficacy of serum NFL as a biomarker of neuroaxonal damage emerged initially in multiple sclerosis, where it serves for prognosis and treatment monitoring [10,11]. Currently, there is growing scientific evidence associating serum NFL with other neurodegenerative diseases [12] and peripheral neuropathies in humans [13–25] and animals [26–28]. However, evidence of a potential association between NFL and DSPN is scarce. One recent preliminary study indicated inverse correlations between serum NFL and some nerve function measures in individuals with type 2 diabetes who had a known diabetes duration of 3 years or less [29]. An earlier study reported higher blood NFL (also known as NEFL) mRNA levels in people with impaired glucose tolerance and/ or impaired fasting glucose and peripheral neuropathy than those without [30]. However, whether serum NFL is associated with prevalent DSPN in individuals with type 1 or type 2 diabetes of a very short known duration remains unknown.
Therefore, the primary aim of the present study was to investigate associations of serum NFL with prevalent DSPN and peripheral nerve function in recently diagnosed type 1 and type 2 diabetes. We hypothesised that higher serum NFL levels are associated with prevalent DSPN and nerve dysfunction. Given that we measured serum NFL with proximity extension assay technology in a 92-biomarker panel, the secondary aim of our study was to explore potential associations of other biomarkers in this panel with DSPN.
Methods
Study design and study population The German Diabetes Study (GDS) is an ongoing observational prospective study that evaluates the natural course of recently diagnosed diabetes and explores prognostic factors and mechanisms leading to diabetes-related complications [31]. Individuals aged 18–69 years at the baseline examination with a known diabetes duration of less than 1 year are eligible to participate, while individuals with overt neurological diseases, such as multiple sclerosis, dementia or psychiatric disorders, are excluded. Diabetes is diagnosed according to the ADA criteria [32]. At baseline, all participants undergo a comprehensive examination consisting of clinical tests, a face-to-face interview, standardised written questionnaires and detailed laboratory measurements.
The GDS is conducted according to the Declaration of Helsinki, approved by the ethics committee of Heinrich Heine University, Düsseldorf, Germany (ref. 4508) and was registered with ClinicalTrials.gov (registration no. NCT01055093). All participants provided written informed consent.
This cross-sectional analysis focused on 503 participants recruited consecutively from 2005 to 2011. From these 503 participants, we excluded 51 participants with missing data on one of the neurological variables used to define DSPN, two participants with diabetes forms other than type 1 or type 2 diabetes and 36 participants with missing covariates, leaving 423 participants with complete data for analysis (ESM Fig. 1).
Measurement of serum NFL NFL was measured in serum of fasting participants at baseline using the Olink Target 96 Neuro Exploratory multiplex assay panel (Olink Proteomics, Uppsala, Sweden; NFL Uniprot ID: P07196 and NFL Olink ID: OID05206) based on proximity extension assay technology. The relative concentration of serum NFL is expressed as normalised protein expression (NPX) values, which are comparable in their distribution to log2-transformed protein concentrations. A detailed description of all 92 biomarkers in the panel is given in ESM Table 1; data cleaning steps that left 60 biomarkers, in addition to NFL, for exploratory analysis are described in ESM Fig. 2. We first excluded 29 biomarkers because of missing data for ≥25%. Then, we further excluded two biomarkers because of inter-assay CV >25%.
Assessment of peripheral neuropathy All participants underwent nerve conduction studies (NCSs) and quantitative sensory testing (QST) as previously described [33, 34]. Motor nerve conduction velocity (MNCV) was measured in the peroneal, median and ulnar nerves, while sensory nerve conduction velocity (SNCV) and sensory nerve action potential (SNAP) were measured in the sural, median and ulnar nerves. All nerve stimulations were performed using surface electrodes (Nicolet VikingQuest; Natus Medical, San Carlos, CA, USA) after warming up feet and lower legs to ensure that skin temperature was 33–34°C. QST was evaluated by thermal detection thresholds (TDTs) to warm and cold stimuli at the thenar eminence and dorsum of the foot using the method of limits (TSA-II NeuroSensory Analyzer; Medoc, Ramat Yishai, Israel). Neurological examination was performed using the Neuropathy Disability Score (NDS) for neuropathic signs and the Neuropathy Symptom Score (NSS) for neuropathic symptoms [35]. Stages of DSPN were defined, according to the Toronto Consensus criteria [36], as subclinical, confirmed asymptomatic and confirmed symptomatic as previously described [33].
Covariates Body weight (kg), waist circumference (cm) and height (m) were measured at enrolment. Information on known diabetes duration (days), presence of chronic diseases, use of non-steroidal anti-inflammatory drugs (NSAIDs) (yes/ no) and lipid-lowering drugs (yes/no) was obtained during a face-to-face interview. Hypertension was defined as either systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or use of antihypertensive medication. Self-reported myocardial infarction, peripheral arterial occlusive disease, cerebrovascular disease or stroke was used to define the presence of CVD. Total cholesterol and HbA1c were measured according to standardised laboratory procedures in blood samples collected at baseline after overnight fasting [31]. Diabetes-related autoantibodies were measured for each participant. The eGFR was calculated from serum creatinine and cystatin C using the Chronic Kidney Disease Epidemiology (CKD-EPI) equation [37].
Statistical analyses Data are presented as median (25th, 75th percentiles), mean ± SD, or percentages in descriptive statistics. Differences in characteristics according to DSPN status were tested with generalised linear regression analyses allowing for different group variances using the SAS procedure GLIMMIX and with the χ2 test, while differences in serum NFL levels between the two groups were tested with ANCOVA to allow adjustment for age at diagnosis. In addition, differences in means and 95% CIs were also calculated. Spearman’s rank correlation tests estimated the non-adjusted and age-adjusted correlations of serum NFL with demographic and metabolic variables.
In primary analyses, we assessed the associations of serum NFL with DSPN (primary endpoint) and with nerve function measures (exploratory secondary endpoints). First, the association of serum NFL (independent variable, by 1-NPX increase, by 1-SD increase and by tertiles) with DSPN (binary dependent variable) was assessed using Poisson regression models with a robust error variance. Model 1 was adjusted for sex and age at diagnosis. Model 2 was additionally adjusted for waist circumference, height, HbA1c, known diabetes duration, diabetes type, eGFR, total cholesterol, hypertension, CVD, lipid-lowering drugs and NSAIDs. Based on our previous studies, these covariates were defined a priori as covariates that may affect nerve conduction [38, 39]. Associations were estimated with RRs of DSPN and their corresponding 95% CIs. Receiver operating characteristic (ROC) curves were used to assess the predictive performance of serum NFL. We conducted the following sensitivity analyses to test the robustness of our results: (1) we substituted waist circumference and height with BMI; (2) we excluded individuals with type 1 diabetes; and (3) we excluded participants with prevalent self-reported CVD (n=10). In addition, we tested for interaction with diabetes type. Second, the associations of serum NFL with MNCVs and SNCVs were assessed using multivariable linear regression models, while associations with SNAPs and TDTs were assessed using quantile regression models, which do not make assumptions about the residual distribution. These analyses were adjusted for the same covariates as in the Poisson regression models, and associations were estimated with β estimates and their corresponding 95% CIs. In addition, for each participant, individual MNCVs, SNCVs and SNAPs were standardised and summarised in sum scores, which were used as additional secondary outcomes analysed using multivariable linear regression models. Z scores were calculated by subtracting the mean value of nerve conduction velocity (NCV) in the study population from the value in the individual and dividing the result by the SD. We constructed an ‘MNCV sum score’ based on MNCVs, an ‘SNCV sum score’ based on SNCVs, and a ‘total NCV sum score’ based on MNCVs, SNCVs and SNAPs. The sum scores combine information about the NCVs of different nerves by giving equal weight to each nerve, allowing for a more comprehensive assessment of nerve function in an individual [39,40]. Primary analyses addressing a pre-planned hypothesis on NFL with primary and secondary exploratory endpoints were not adjusted for multiple testing.
In a secondary hypothesis-free exploratory analysis, all analyses mentioned above for NFL were performed for the remaining 60 biomarkers of the Olink Target 96 Neuro Exploratory multiplex assay. These hypothesis-free analyses were adjusted for multiple testing with the Bonferroni method (a recommended approach when many tests are carried out without pre-planned hypotheses) [41] and a Bonferronicorrected p<0.0008 (0.05/61) indicated significant associations.
All statistical analyses were carried out with SAS version 9.4 (SAS Institute, Cary, NC, USA), and p values <0.05 were considered indicators of a statistically significant correlation, difference or association unless otherwise stated. The visualisation was carried out with RStudio version 4.0.5 (https:// posit.co/download/rstudio-desktop).
Results
Participants’ characteristics Participants with different DSPN severity stages (subclinical [n=41], confirmed asymptomatic [n=11] and confirmed symptomatic [n=14]) were merged to create an overall DSPN group (n=66, 16%), which was compared with participants without DSPN (n=357, 84%).
Table 1 shows the demographic and clinical characteristics of the study population overall (n=423) and stratified by DSPN status. Participants with DSPN were more likely to be men, to be older, and to have higher waist circumference and height than individuals without DSPN. They were also more likely to have type 2 diabetes, a shorter known diabetes duration and use lipid-lowering drugs. However, there was no evidence for differences in HbA1c, cholesterol levels or proportion of individuals with hypertension, CVD and use of NSAIDs between those with and without DSPN. Serum NFL levels (median [25th percentile, 75th percentile]) were higher in participants with DSPN (4.0 [3.6, 4.5]) compared with those without DSPN (3.7 [3.2, 4.0], p<0.0001) (Table 1 and Fig. 1), and higher in each DSPN stage compared with the group without DSPN despite the small sample size (ESM Fig. 3).
Serum NFL levels were positively correlated with age at diagnosis (r=0.61, p<0.0001) (ESM Fig. 4) but correlations with BMI, waist circumference, height, HbA1c, total cholesterol and eGFR varied before and after adjustment for age (ESM Table 2).
Association of serum NFL with DSPN Table 2 shows the association between serum NFL and prevalent DSPN. In model 1, adjusted for sex and age at diagnosis, higher serum NFL levels were positively associated with prevalent DSPN (RR [95% CI] per 1-NPX increase, 1.94 [1.51, 2.49]; p<0.0001). This positive association remained constant in model 2 (the fully adjusted model) (RR [95% CI] 1.92 [1.50, 2.45]; p<0.0001). Similarly, participants in the second and third tertiles of serum NFL showed higher adjusted RRs for prevalent DSPN compared with those in the lowest tertile (RR [95% CI] 2.63 [1.31, 5.29] for tertile 2 and 4.28 [1.50, 12.17] for tertile 3; ptrend=0.007). ESM Fig. 5 shows that the AUC of serum NFL for DSPN as outcome was 0.66 (95% CI 0.59, 0.74; p<0.0001).
Sensitivity analyses The association of serum NFL levels with prevalent DSPN remained consistent when waist circumference and height were substituted with BMI (RR [95% CI] per 1-NPX increase 1.88 [1.47, 2.40]) and when the analyses were restricted to participants with type 2 diabetes (RR [95% CI] 1.96 [1.51, 2.54]). These associations remained robust when the analysis was repeated after excluding participants with prevalent CVD (RR [95% CI] 1.87 [1.46, 2.39]). Interaction by diabetes type was not significant (p=0.99).
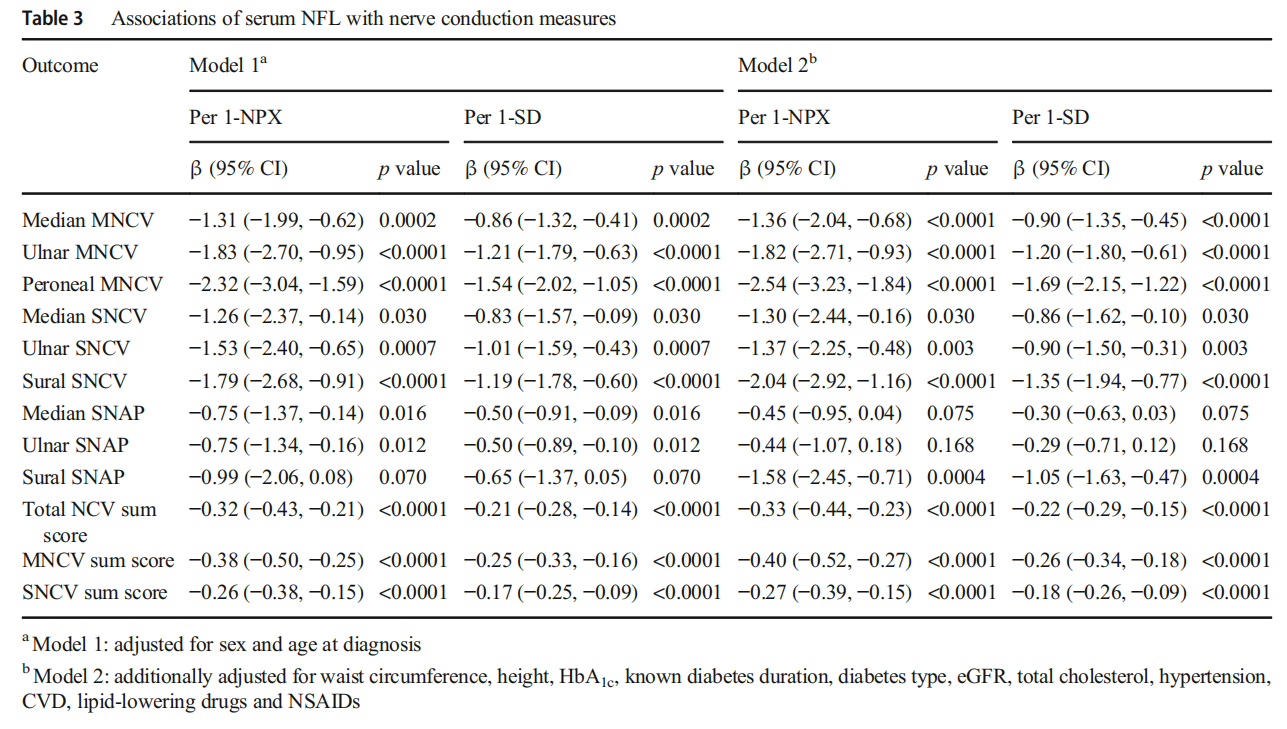
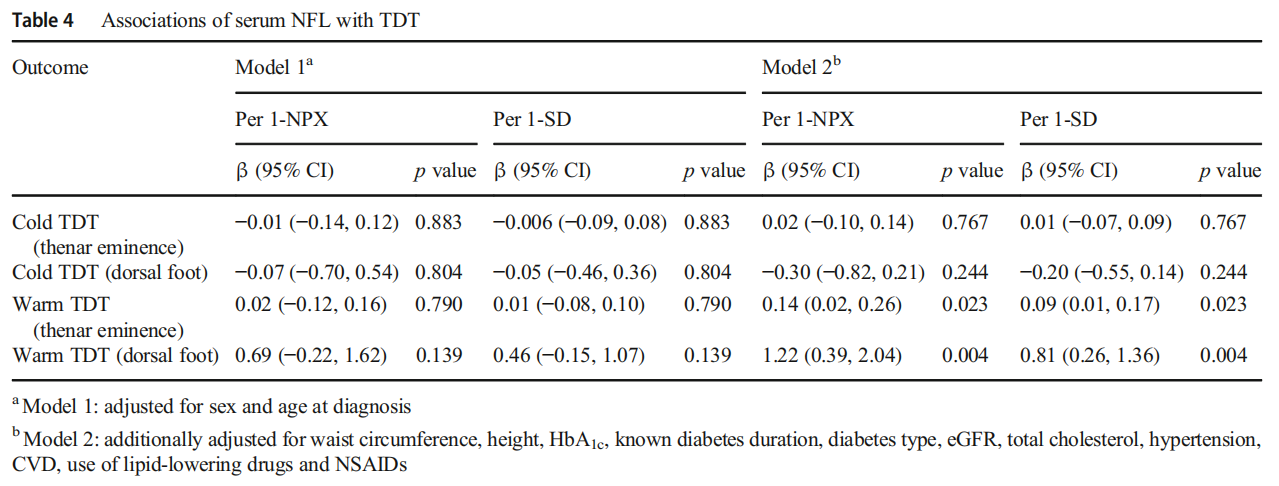
Associations of serum NFL with peripheral nerve function tests Table 3 displays the associations of serum NFL with nerve conduction measures. Higher serum NFL levels were associated with slower MNCV (all p<0.0001) and SNCV (all p≤0.03) in all nerves and lower NCV sum scores (all p<0.0001). These associations were observed in model 1 and remained consistent in model 2. The highest estimates were found for peroneal MNCV and sural SNCV. In addition, higher serum NFL levels were associated with lower sural SNAP (p=0.0004) only in model 2. Table 4 displays the associations of serum NFL with TDT. Higher serum NFL levels were only associated with higher TDT to warm stimuli on the hand and foot in model 2 (p=0.023 and p=0.004 for hand and foot, respectively).
Exploratory analyses ESM Fig. 6 shows that correlations between biomarkers were almost all positive (r values ranged between 0.1 and 0.5) and ESM Fig. 7 shows that correlations between most biomarkers and age, BMI, waist circumference and total cholesterol were predominantly positive. In contrast, correlations between biomarkers and eGFR were mainly negative. Most biomarkers, except cadherin (CDH)-15, secreted frizzled-related protein 1 (SFRP1) and signal recognition particle 14 kDa protein (SRP14), did not show differences in their expression levels when comparing individuals with and without DSPN (p>0.05) (ESM Table 3).
Associations of biomarkers with prevalent DSPN are reported in ESM Table 4. Only serum SFRP1 was positively associated with prevalent DSPN in model 1 and model 2 but this association did not remain significant after adjustment for multiple testing. β estimates for the associations of biomarkers with MNCVs, SNCVs and NCV sum scores are shown in ESM Fig. 8 (β estimates by 1-NPX increase) and in ESM Fig.9 (β estimates by 1-SD increase). Most associations were inverse. After full adjustment for covariates, eight biomarkers were associated with MNCV in at least one motor nerve and six biomarkers were associated with SNCV in at least one sensory nerve (p<0.05). In particular, CDH17 and disintegrin and metalloproteinase domain-containing protein 15 (ADAM15) showed inverse associations with the three sensory nerves investigated. Proline-rich Akt 1 substrate 1 (AKT1S1) was the only biomarker inversely associated with MNCV and SNCV. However, these associations were abolished when multiple testing was taken into account.
Discussion
The results of this cross-sectional study in individuals recently diagnosed with type 1 and type 2 diabetes from the GDS baseline cohort demonstrated associations between higher serum NFL levels and prevalent DSPN. In addition, we showed the association of higher serum NFL levels with large myelinated fibre dysfunction, evident by slower MNCV and SNCV as well as a lower SNAP. Moreover, higher serum NFL levels were associated with elevated TDTs to warm rather than cold stimuli, indicating that small unmyelinated Cfibres, rather than thinly myelinated Aδ-fibres, contributed to this relationship. These associations were independent of age and other covariables, robust to sensitivity analyses, and aligned with our hypothesis that serum NFL is a promising biomarker to indicate early peripheral nerve dysfunction due to DSPN. However, this study did not reveal any associations between other neurological biomarkers and DSPN in exploratory analyses.
Our study is the first to show that high serum NFL levels are associated with an almost fourfold higher prevalence of DSPN. We measured serum NFL with a sensitive method and defined DSPN using the Toronto Consensus criteria. Additionally, we adjusted the associations for relevant confounders. When analysing NCS data separately, associations were more pronounced in the peroneal motor and sural sensory nerves than in the median and ulnar motor and sensory nerves. This pattern indicates a relatively higher degree of myelin damage and more intense axonal damage in lower limb nerves than in upper limb nerves. Such a finding is plausible for the following reasons: (1) the longer lower limb axons are more vulnerable to injury than upper limb axons [4]; (2) substantial evidence suggests that the earliest nerve damage occurs in the sural nerve early after diabetes diagnosis [42]; and (3) electrophysiological measures acquired in the sural sensory and peroneal motor nerves are considered the most sensitive NCS by which to detect early large fibre dysfunction in diabetes [43] and represent the first-line tests by the American Academy of Neurology [44].
Our findings considerably extend those of a previous epidemiological study reporting inverse correlations between serum NFL and NCV in the peroneal motor but not the sural sensory nerve in individuals with type 2 diabetes [29]. However, that study reported only Spearman’s correlations and did not estimate the associations between serum NFL and NCVs in multivariable models, possibly introducing bias due to confounding. In contrast, our study adjusted for multiple confounders. In addition, the previous study’s participants included only individuals with type 2 diabetes who were relatively older (age 35–85 years) and had a longer known diabetes duration (up to 3 years) compared with our study, which included participants with type 1 diabetes and type 2 diabetes, who were relatively younger (median age 47.7 years) and had a median known diabetes duration of 6 months. Therefore, our study provides the first comprehensive analysis of the associations between serum NFL and nerve function in individuals recently diagnosed with diabetes.
Notably, the observed associations between serum NFL and sural sensory nerve function are consistent with sural nerve biopsies indicating a positive correlation between higher serum NFL levels and axonal loss in older individuals with peripheral neuropathy of different aetiologies (6% diabetic) [45]. Thus, both electrophysiological and pathological findings reinforce the utility of serum NFL in quantifying axonal degeneration.
Though NFL is known as a biomarker of axonal loss, we detected more robust associations with NCV (typically regarded as an indicator of myelin damage) than with SNAP (typically regarded as an indicator of axonal loss). Evidence indicates that demyelination can exist in people with diabetes with and without symptomatic DSPN, but the axonal loss is linked to the appearance of symptoms [46]. Since only 14 out of 66 individuals with DSPN in our sample were symptomatic, this low prevalence of symptomatic DSPN might explain our study’s less pronounced associations between NFL and SNAP compared with the more pronounced associations with NCV. Nevertheless, the observed associations of NFL with both NCV and SNAP, though to a different extent, could suggest that NFL can be considered a biomarker of overall nerve injury (axonal loss and demyelination) rather than solely axonal loss. Others have shown increased NFL levels in individuals with polyneuropathies of different aetiologies irrespective of the associated pattern (demyelinating, axonal or both) [22], strengthening the suggestion that increased systemic NFL levels may be an indicator of either axonal damage, demyelination or both.
Associations between serum NFL and nerve conduction were more robust and consistent than associations between NFL and sensory nerve function, although large nerve fibres represent only a tiny fraction of nerve fibres. This difference is likely due to the abundant expression of NFL in large fibres where it is needed to increase the axonal diameter and speed up nerve conduction [10], unlike small sensory nerves with minimal nerve conduction that do not express high NFL levels. Another reason for this difference is that NCSs represent a more objective and robust instrument to estimate DSPN than QST [47]. Consequently, NCS-driven measures of nerve function in large nerves might be more relevant in this context than QST-driven measures in small nerves. In contrast to our study, which found associations of serum NFL with TDT to warm but not cold stimuli, a previous study measuring serum NFL with the Simoa Technology showed correlations with both warm and cold stimuli [29], although that study reported only non-adjusted correlations.
This present study demonstrated higher age-independent serum NFL levels in individuals with DSPN than in those without DSPN shortly after a diabetes diagnosis. A previous study in individuals with known diabetes duration of less than 3 years did not find a difference in serum NFL levels between individuals with and without DSPN [29]. However, the same study found a difference in serum NFL levels between individuals with DSPN and healthy control individuals. In another study, circulatory NFL mRNA was higher in individuals with DSPN than in those without DSPN [30]. It is worth noting that elevated serum NFL levels have been reported in peripheral neuropathies other than DSPN [13, 15, 17, 22, 25].
Substantial evidence associates higher serum NFL levels with various neurodegenerative diseases of the peripheral and central nervous systems [12]. Therefore, serum NFL is not a DSPN-specific biomarker and cannot be used as a biomarker for the diagnosis of DSPN. However, if the association between serum NFL and DSPN reported in our study is validated in other studies, future research should investigate the clinical utility of serum NFL as a biomarker for DSPN monitoring, given that a periodic serum NFL measurement is noninvasive and can be accessible in daily clinical care. Additionally, future studies might then investigate the potential of serum NFL as a candidate surrogate endpoint in phase II trials evaluating new therapies for DSPN, given that laboratory-measured biomarkers are objective, reproducible and not subject to inter-observer variability.
Exploratory analysis revealed inverse associations between some neurological biomarkers (e.g. ADAM15, involved in wound healing) and NCVs; these associations were abolished after adjustment for multiple testing, likely because Bonferroni correction is a conservative approach. It is also plausible that biomarkers reflecting nerve repair processes and axon development have a limited role in recent-onset diabetes. Thus, future studies, including participants with more prolonged diabetes, may reveal more biomarkers associated with nerve injury.
This study has several strengths. First, we included relatively young individuals with a median known diabetes duration of 6 months and good overall health status. This selection allowed analyses of associations without the confounding effect of late diabetes-related and ageing-related comorbidities. Second, neurophysiological testing targeted both large and small fibre functions. Third, our statistical analysis was comprehensively adjusted for covariates and associations were assessed for different outcomes modelled as a binary variable, continuous nerve function in single nerves and sum scores. Our study includes the following limitations: (1) the cross-sectional design precludes us from knowing the predictive value of serum NFL; (2) the inclusion of individuals with wellcontrolled diabetes for whom the extent of nerve damage is lower than individuals with less-controlled diabetes; (3) the predominance of subclinical DSPN prevents us from assessing the associations between serum NFL and DSPN stages; and (4) the lack of statistical power to stratify the analyses by diabetes type. However, as the interaction between serum NFL and diabetes type was not significant, there was no evidence for a difference in the observed associations between type 1 and type 2 diabetes. In addition, biomarkers were measured with the highly sensitive proximity extension assay technology. Though protein levels were expressed as NPX values rather than absolute concentrations, this aspect did not impact our findings. Finally, our study population consists of mainly German individuals with short known diabetes duration. Thus, our findings cannot be generalisable to other ethnicities and individuals with longer diabetes duration.
Conclusion Our study indicates that higher serum NFL levels are associated with prevalent DSPN and nerve dysfunction in recently diagnosed diabetes. We advocate serum NFL as a novel blood-based biomarker of nerve injury in DSPN. However, the potential clinical utility of serum NFL as a monitoring biomarker for DSPN in routine clinical practice and as a biomarker in clinical trials needs to be investigated in future studies.
Supplementary Information The online version of this article (https://doi. org/10.1007/s00125-022-05846-8) contains peer-reviewed but unedited supplementary material..
Acknowledgements We appreciate the contribution of all study participants. The authors thank the staff of the Clinical Research Center at the Institute for Clinical Diabetology, German Diabetes Center, for their excellent work. Data from this study was presented at the ADA congress on 3–6 June 2022 and the Central European Diabetes Association conference on 23–25 June 2022.
The GDS Group consists of H. Al-Hasani, V. Burkart, A. E. Buyken, G. Geerling, C. Herder, A. Icks, K. Jandeleit-Dahm, J. Kotzka, O. Kuss, E. Lammert, W. Rathmann, V. Schrauwen-Hinderling, J. Szendroedi, S. Trenkamp, D. Ziegler and M. Roden (speaker).
Data availability
The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, data can be requested through an individual project agreement with the Steering Committee of the GDS (speaker: M. Roden, 该Email地址已收到反垃圾邮件插件保护。要显示它您需要在浏览器中启用JavaScript。).
Funding
Open Access funding enabled and organized by Projekt DEAL. The German Diabetes Study (GDS) was initiated and financed by the German Diabetes Center (DDZ), which is funded by the German Federal Ministry of Health (Berlin, Germany), the Ministry of Culture and Science of the state of North Rhine-Westphalia (Düsseldorf, Germany) and grants from the German Federal Ministry of Education and Research (Berlin, Germany) to the German Center for Diabetes Research e.V. (DZD). This study was funded by a grant from DZD to HM and SMH. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Authors’ relationships and activities CH received a research grant from Sanofi-Aventis outside the submitted work and is Associate Editor of Diabetologia. WR reports receiving consulting fees for attending educational sessions or advisory boards from AstraZeneca, BoehringerIngelheim and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work. MR received fees as a member of advisory boards or speaker from Allergan, BoehringerIngelheim Pharma, Bristol-Myers Squibb, Eli Lilly, Fishawack Group, Gilead Sciences, Novartis Pharma, Intercept Pharma, Inventiva, Novo Nordisk, AstraZeneca, Sanofi US, Prosciento, Targer RWE, Kenes Group, Target NASH and Terra Firma and has been involved with clinical trial research for Boehringer-Ingelheim, Danone Nutricia Research and Sanofi-Aventis, all outside the submitted work. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
HM and SMH acquired funding. HM had the idea for this research work. HM and CH designed the study. AS, DZ, AP, GJB, SMH, CH, OPZ, VB, JS, WR, ST and MR contributed data. HM drafted the analysis plan and performed the statistical analysis. CH, KS and OK contributed to the statistical analysis. CH, DZ and MR contributed to data interpretation. HM wrote the manuscript. CH, DZ and MR contributed to the draft of the manuscript. All authors reviewed and edited the manuscript and approved its submission. HM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
References
1. Ziegler D, Papanas N, Schnell O et al (2021) Current concepts in the management of diabetic polyneuropathy. J Diabetes Investig 12(4):464–475. https://doi.org/10.1111/jdi.13401
2. Boulton AJ, Kempler P, Ametov A, Ziegler D (2013) Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev 29(5):327–333. https://doi.org/10.1002/dmrr.2397
3. Ziegler D, Tesfaye S, Spallone V et al (2021) Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: International expert consensus recommendations. Diabetes Res Clin Pract 186:109063. https://doi.org/10.1016/j. diabres.2021.109063
4. Pop-Busui R, Boulton AJ, Feldman EL et al (2017) Diabetic neuropathy: a position statement by the american diabetes association. Diabetes Care 40(1):136–154. https://doi.org/10.2337/dc16-2042
5. Callaghan BC, Gallagher G, Fridman V, Feldman EL (2020) Diabetic neuropathy: what does the future hold? Diabetologia 63(5):891–897. https://doi.org/10.1007/s00125-020-05085-9
6. Malik RA (2014) Why are there no good treatments for diabetic neuropathy? Lancet Diabetes Endocrinol 2(8):607–609. https://doi. org/10.1016/S2213-8587(14)70067-1
7. Carrington AL, Shaw JE, Van Schie CH, Abbott CA, Vileikyte L, Boulton AJ (2002) Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care 25(11):2010–2015. https://doi.org/10.2337/diacare. 25.11.2010
8. Bomont P (2021) The dazzling rise of neurofilaments: physiological functions and roles as biomarkers. Curr Opin Cell Biol 68:181–191.https://doi.org/10.1016/j.ceb.2020.10.011
9. Sainio MT, Rasila T, Molchanova SM et al (2021) Neurofilament light regulates axon caliber, synaptic activity, and organelle trafficking in cultured human motor neurons. Front Cell Dev Biol 9:820105. https://doi.org/10.3389/fcell.2021.820105
10. Gafson AR, Barthélemy NR, Bomont P et al (2020) Neurofilaments: neurobiological foundations for biomarker applications. Brain 143(7):1975–1998. https://doi.org/10.1093/brain/ awaa098
11. Ferreira-Atuesta C, Reyes S, Giovanonni G, Gnanapavan S (2021) The evolution of neurofilament light chain in multiple sclerosis. Front Neurosci 15:642384. https://doi.org/10.3389/fnins.2021. 642384
12. Gordon BA (2020) Neurofilaments in disease: what do we know?Curr Opin Neurobiol 61:105–115. https://doi.org/10.1016/j.conb. 2020.02.001
13. Altmann P, De Simoni D, Kaider A et al (2020) Increased serum neurofilament light chain concentration indicates poor outcome in Guillain-Barre syndrome. J Neuroinflammation 17(1):86. https:// doi.org/10.1186/s12974-020-01737-0
14. Bischof A, Manigold T, Barro C et al (2018) Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann Rheum Dis 77(7):1093–1094. https://doi.org/10.1136/ annrheumdis-2017-212045
15. Fukami Y, Iijima M, Koike H, Yamada S, Hashizume A, Katsuno M (2021) Association of serum neurofilament light chain levels with clinicopathology of chronic inflammatory demyelinating polyneuropathy, including NF155 reactive patients. J Neurol 268(10):3835–3844. https://doi.org/10.1007/s00415-021-10537-2
16. Godelaine J, De Schaepdryver M, Bossuyt X, Van Damme P, Claeys KG, Poesen K (2021) Prognostic value of neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. Brain Commun 3(1):fcab018. https://doi.org/10.1093/braincomms/ fcab018
17. Hayashi T, Nukui T, Piao JL et al (2021) Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. Brain Behav 11(5):e02084. https://doi.org/10.1002/brb3.2084
18. Huehnchen P, Schinke C, Bangemann N et al (2022) Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy. JCI Insight 7(6):e154395. https://doi.org/10. 1172/jci.insight.154395
19. Kim S-H, Choi MK, Park NY et al (2020) Serum neurofilament light chain levels as a biomarker of neuroaxonal injury and severity of oxaliplatin-induced peripheral neuropathy. Sci Rep 10(1):7995. https://doi.org/10.1038/s41598-020-64511-5
20. Louwsma J, Brunger AF, Bijzet J et al (2021) Neurofilament light chain, a biomarker for polyneuropathy in systemic amyloidosis. Amyloid 28(1):50–55. https://doi.org/10.1080/13506129.2020. 1815696
21. Maia LF, Maceski A, Conceição I et al (2020) Plasma neurofilament light chain: an early biomarker for hereditary ATTR amyloid polyneuropathy. Amyloid 27(2):97–102. https://doi.org/10.1080/ 13506129.2019.1708716
22. Mariotto S, Farinazzo A, Magliozzi R, Alberti D, Monaco S, Ferrari S (2018) Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J Peripher Nerv
Syst 23(3):174–177. https://doi.org/10.1111/jns.12279
23. Millere E, Rots D, Simrén J et al (2021) Plasma neurofilament light chain as a potential biomarker in Charcot-Marie-Tooth disease. Eur J Neurol 28(3):974–981. https://doi.org/10.1111/ene.14689
24. Sandelius Å, Zetterberg H, Blennow K et al (2018) Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90(6):e518–e524. https://doi.org/10. 1212/WNL.0000000000004932
25. van Lieverloo GGA, Wieske L, Verhamme C et al (2019) Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 24(2):187–194. https://doi. org/10.1111/jns.12319
26. Meregalli C, Fumagalli G, Alberti P et al (2018) Neurofilament light chain as disease biomarker in a rodent model of chemotherapy induced peripheral neuropathy. Exp Neurol 307:129–132. https:// doi.org/10.1016/j.expneurol.2018.06.005
27. Meregalli C, Fumagalli G, Alberti P et al (2020) Neurofilament light chain: a specific serum biomarker of axonal damage severity in rat models of chemotherapy-induced peripheral neurotoxicity. Arch Toxicol 94(7):2517–2522. https://doi.org/10.1007/s00204- 020-02755-w
28. Sano T, Masuda Y, Yasuno H, Shinozawa T, Watanabe T, Kakehi M (2021) Blood neurofilament light chain as a potential biomarker for central and peripheral nervous toxicity in rats. Toxicol Sci 185(1):10–18. https://doi.org/10.1093/toxsci/kfab122
29. Morgenstern J, Groener JB, Jende JME et al (2021) Neuronspecific biomarkers predict hypo- and hyperalgesia in individuals with diabetic peripheral neuropathy. Diabetologia 64(12):2843–2855. https://doi.org/10.1007/s00125-021-05557-6
30. Celikbilek A, Tanik N, Sabah S et al (2014) Elevated neurofilament light chain (NFL) mRNA levels in prediabetic peripheral neuropathy. Mol Biol Rep 41(6):4017–4022. https://doi.org/10.1007/ s11033-014-3270-y
31. Szendroedi J, Saxena A, Weber KS et al (2016) Cohort profile: the German Diabetes Study (GDS). Cardiovasc Diabetol 15:59. https:// doi.org/10.1186/s12933-016-0374-9
32. American Diabetes Association (2018) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 41(Suppl 1):S13–s27. https://doi.org/10.2337/dc18-S002
33. Ziegler D, Bonhof GJ, Strom A et al (2021) Progression and regression of nerve fibre pathology and dysfunction early in diabetes over 5 years. Brain 144(10):3251–3263. https://doi.org/10.1093/brain/ awab330
34. Strom A, Kaul K, Brüggemann J et al (2017) Lower serum extracellular superoxide dismutase levels are associated with polyneuropathy in recent-onset diabetes. Exp Mol Med 49(11):e394. https://doi.org/10.1038/emm.2017.173
35. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH (1993) A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 36(2):150–154. https://doi.org/10.1007/BF00400697
36. Dyck PJ, Albers JW, Andersen H et al (2011) Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 27(7):620-628. https://doi.org/10.1002/dmrr.1226
37. Inker LA, Schmid CH, Tighiouart H et al (2012) Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367(1):20–29. https://doi.org/10.1056/ NEJMoa1114248
38. Herder C, Kannenberg JM, Carstensen-Kirberg M et al (2018) A systemic inflammatory signature reflecting cross talk between innate and adaptive immunity is associated with incident polyneuropathy: KORA F4/FF4 study. Diabetes 67(11):2434–2442. https://doi.org/10.2337/db18-0060
39. Schamarek I, Herder C, Nowotny B et al (2016) Adiponectin, markers of subclinical inflammation and nerve conduction in individuals with recently diagnosed type 1 and type 2 diabetes. Eur J Endocrinol 174(4):433–443. https://doi.org/10.1530/EJE-15-1010
40. Maalmi H, Wouters K, Savelberg H et al (2021) Associations of cells from both innate and adaptive immunity with lower nerve conduction velocity: the Maastricht Study. BMJ Open Diabetes Res Care 9(1):e001698. https://doi.org/10.1136/bmjdrc-2020- 001698
41. Armstrong RA (2014) When to use the Bonferroni correction. Ophthalmic Physiol Opt 34(5):502–508. https://doi.org/10.1111/ opo.12131
42. Cappellari A, Airaghi L, Capra R et al (2005) Early peripheral nerve abnormalities in impaired glucose tolerance. Electromyogr Clin Neurophysiol 45(4):241–244
43. Vas PR, Sharma S, Rayman G (2015) Distal sensorimotor neuropathy: improvements in diagnosis. Rev Diabet Stud 12(1-2):29–47. https://doi.org/10.1900/rds.2015.12.29
44. England JD, Gronseth GS, Franklin G et al (2005) Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 64(2):199–207. https://doi.org/10.1212/01.wnl.0000149522.32823.ea
45. Mariotto S, Carta S, Bozzetti S et al (2020) Sural nerve biopsy: current role and comparison with serum neurofilament light chain levels. J Neurol 267(10):2881–2887. https://doi.org/10.1007/ s00415-020-09949-3
46. Valls-Canals J, Povedano M, Montero J, Pradas J (2002) Diabetic polyneuropathy. Axonal or demyelinating? Electromyogr Clin Neurophysiol 42(1):3–6
47. Tesfaye S, Boulton AJ, Dyck PJ et al (2010) Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10):2285–2293. https://doi.org/10. 2337/dc10-1303
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the Diabetologia (2023) 66:579–589 by Wound World.