The process of wound healing involves a complex series of events that are interlinked and dependent on local and systemic factors ( Guo and DiPietro, 2010). Recognition and eradication of these factors are fundamental steps in successful wound management.
Negative pressure wound therapy (NPWT), is a wound dressing system that continuously or intermittently applies subatmospheric pressure to the surface of the wound, causing negative pressure, which in turn has positive effects on the wound healing trajectory or cascade. NPWT guidelines suggested pressure between −75 and −125mmHg would provide a moist environment (Wayne et al, 1996; Bryan, 2004; Apelqvist et al, 2017) that is suitable for wound healing. NPWT improves the rate of angiogenesis (Fabian et al, 2000; Chen et al, 2005), endothelial proliferation (Scherer et al, 2008), capillary blood flow (Morykwas et al, 1997; Wackenfors et al, 2004; Chen et al, 2005; Wackenfors et al, 2005), while reducing oedema (Kamolz et al, 2004; Simman et al 2004) and the bacterial burden (Mouës et al, 2004) within the wound. Pressure greater than −125mmHg is known to cause pain (Borgquist et al, 2010).
A case study of 20 patients with infected wounds, using NPWT as an adjunct therapy, showed that NPWT stimulates infection-free scar tissue formation in a short time (Jones et al, 2016). In another pilot study involving 15 patients with infected wounds, NPWT instillation showed a significant decrease in the mean-time to bioburden reduction, wound closure and hospital discharge compared with traditional wet-to-moist wound care (Gabriel et al, 2008). Both of these study outcomes demonstrates NPWT as being able to reduce the need for complex surgical procedures and decrease inpatient care requirements for these complex, infected wounds.
An evaluation of NPWT with low pressure and gauze dressing in a 30 patients prospective cohort study shows 43% achieved at least 50% wound area reduction after four weeks of therapy (Lavery et al, 2014). In a randomised controlled trial (RCT), prophylactic NPWT application to laparotomy wounds after an abdominal surgery minimised the risk of developing surgical site infection (SSI) compared with the control group (O’Leary et al 2017). Efficacy of NPWT in reducing SSI rate is also evident in two other studies with ileostomy closure sites (Cantero et al, 2016; Poehnert et al, 2017).
In acute management of burns, NPWT is proven to reduce ischaemia, oedema formation and wound progression (Morykwas et al, 1999; Kamolz et al 2004; Schrank et al 2004; Molnar et al, 2006). NPWT is used with caution in paediatric patients as their skin thickness and composition (Birchenough et al 2008; Stamatas et al, 2009), body surface area (Passaretti and Billmire, 2003) and formation rates of granulation tissue (Mooney et al, 2000)differs in comparison with adults. However, on suitable paediatric patients, NPWT is convenient to be used as it reduces the frequency of dressing change and allows for greater mobility than some standard dressings (Contractor et al, 2008).
Aim
The aim of this study was to assess the healing of chronic wounds treated by single-use NPWT.
Methodology
Study participants were selected by simple random sampling from a pool of patients who were attending for their routine follow-up visits at the Wound Care Unit in Hospital Kuala Lumpur. During each dressing change, the wound was assessed and cleansed with distilled water. Debridement was done when necessary. The application of NPWT was a simple 3-step procedure (Figure 1), removing the release paper from the dressing and positioning it over the wound bed, connecting the tubing from the pump to the dressing and providing negative pressure treatment through the swing. Patients and caretakers were advised on proper management of pressure ulcers (PU) such as 2-hourly positioning, the use of a proper support surface and good nutritional diet for faster wound healing.
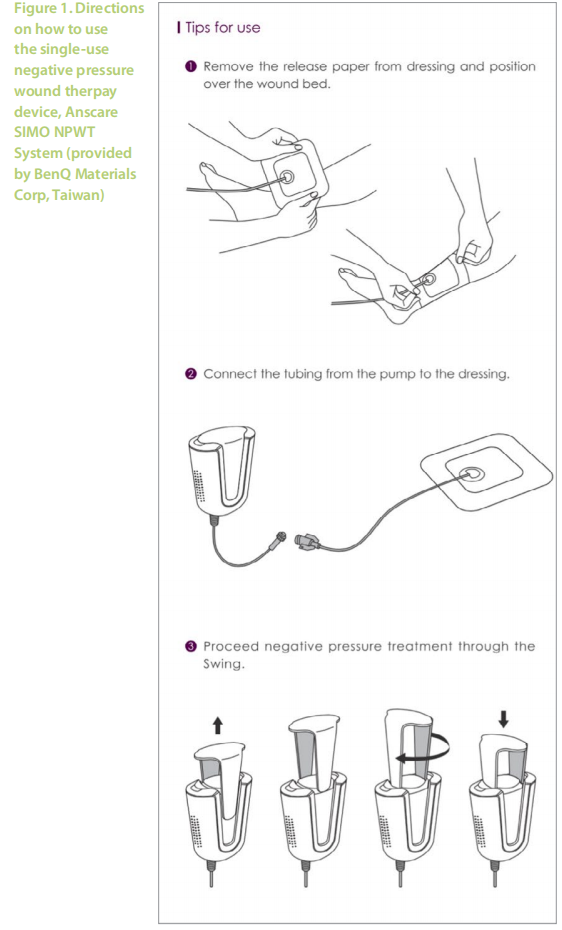
The trial was conducted in accordance with the guidelines set in the Declaration of Helsinki and approved by the hospital review board. Informed consent and permission to use clinical images and case details for publication/research purposes were obtained before starting the study.
Device used in the study
The NPWT used for this case study was Anscare SIMO NPWT System which was provided by BenQ Materials Corp, Taiwan.
Results
We enrolled five patients with chronic wounds of various size and aetiologies, two PU, two abscesses and one post-motor-vehicle accident wound, in this study.
All five cases showed complete wound healing. The two abscess healed on week 6 and 8, while the traumatic wound healed in 10 weeks. Both the sacral and gluteal PU healed on week 28 and week 14 respectively
Case 1
A 62-years-old, Malay gentleman, with underlying diabetes mellitus and hypertension (Case 1). The patient had a perforated appendiceal tumour, which led to a laparotomy, right hemicolectomy, and double barrel stoma in the same month. However, two months postoperative, the patient developed category IV sacral PU. The ulcer size was 2cm x 8.5cm x 5cm on presentation. NPWT treatment was initiated and after 28 weeks of treatment the PU was completely healed.
Case 2
A 60-year-old, Malay gentleman, with underlying diabetes mellitus and hypertension (Case 2). Patient had saucerization (surgical excavation of tissue forming a shallow depression to aid drainage from infected areas of a wound) for the carbuncle over his back under private general practitioner, four months before the start of NPWT. Patient was previously on superoxidised solution, hydrogel and hydrofiber Ag dressing. Once NPWT was started the wound healed after 6 weeks
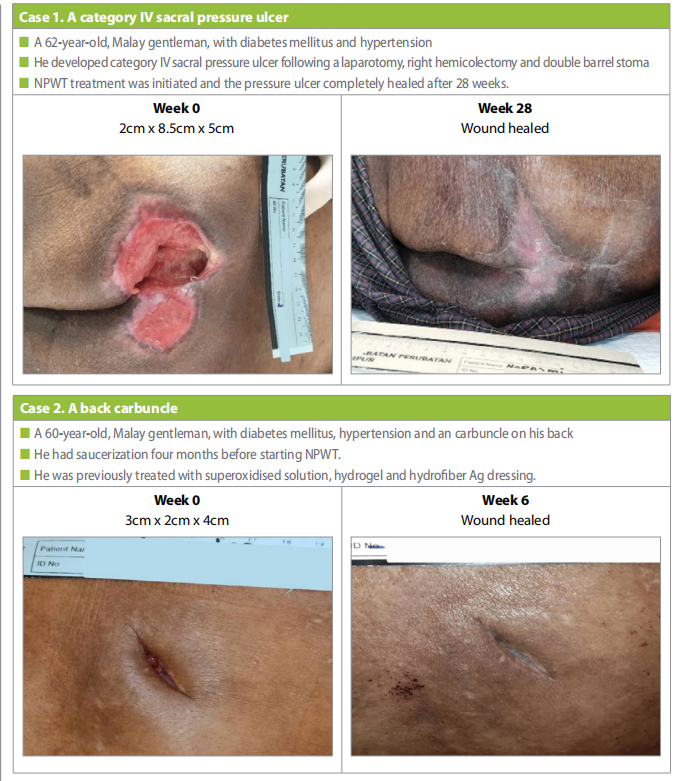
Case 3
A 51-year-old, Indian gentleman, with underlying hypertension, diabetes mellitus and history of a stroke (six months before). He had developed a bilateral gluteal PU due to prolonged intubation in the intensive care unit (Case 3). Initially hydrofiber Ag and polyurethane foam, however after four months it was decided to use NPWT as wound healing progression was slow.
On presentation the PU measure measure 4.5cm x 1.5cm x 3cm, after 14 weeks of NPWT the the ulcer completely healed.
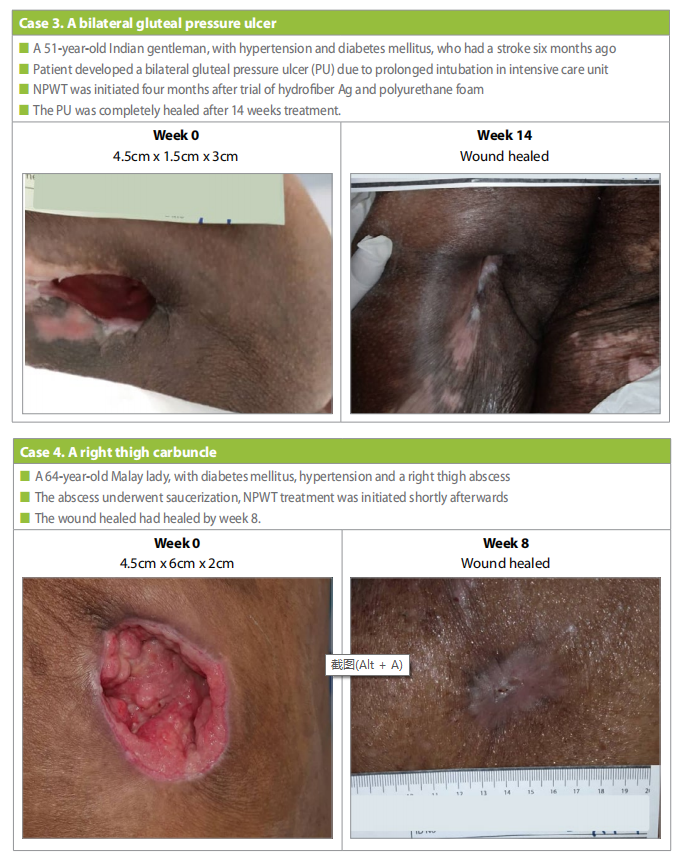
Case 4
A 64-year-old, Malay woman, with underlying diabetes mellitus and hypertension. She had a right thigh carbuncle that was treated with saucerization (Case 4).
NPWT treatment was initiated shortly after the saucerization. At the start of NPWT the wound was 4.5cm x 6cm x 2cm, after 8 weeks the wound was completely healed.
Case 5
A 53-year-old, Malay lady, with no known medical illness. She had a motorbike accident with a deep wound at left shin (Case 5), 7 weeks before initiation of NPWT.
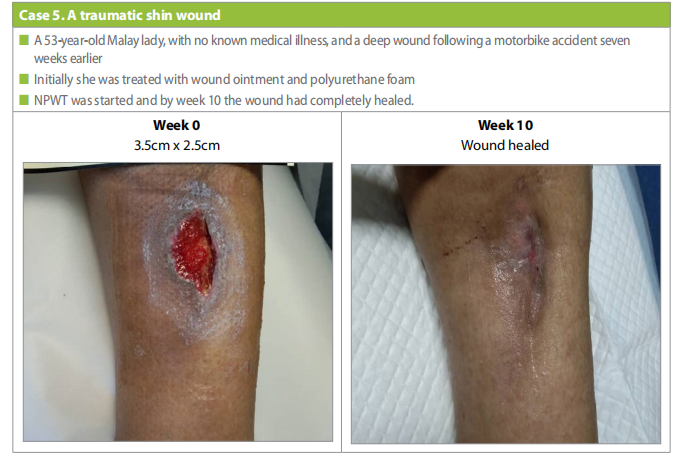
Patient was previously on natural remedy ointment and polyurethane foam before NPWT. As wound healing progression was slow. it was decided to try the single-use NPWT system. On starting tretament the wound size was 3.5cm x 2.5cm after 10 weeks of NPWT the wound has completely healed.
Discussion
Case 3 was a gluteal PU that healed at week 14 due to the site and the good standard of care, including 2-hourly repositioning, a proper support surface and good nutritional diet. Meanwhile, the sacral pressure injury wound took double the time i.e. 28 weeks as the wound area is larger and poor adherence to standard of care.
NPWT increases the rate of angiogenesis (Fabian et al, 2000; Chen et al, 2005), endothelial proliferation (Scherer et al, 2008) as well as reduces oedema (Kamolz et al, 2004) and bacterial burden (Mouës et al, 2004). Hypoxic environment in NPWT increases inflammatory cytokines, hence, stimulating wound healing (Huang et al, 2014; O’Leary et al, 2017).
Patients with limited mobility and heavily exudating wounds used larger mains-operated NPWT in hospitals. The Anscare SIMO NPWT System used in this study is portable, small and lightweight, enabling patients to have continuous NPWT without limiting patient’s mobility. This lightweight, battery-powered units are suitable for ambulatory patients with minimal-to-moderate levels of exudative wound. Anscare SIMO NPWT System is able to promote blood circulation (Morykwas et al. 1997; Wackenfors et al, 2004; Chen et al, 2005; Wackenfors et al, 2005) and exudate management (Wayne et al, 1996; Bryan, 2004; Apelqvist et al 2017) for a better wound healing. This system has a smart indicator for dressing change notification and display of negative pressure status by colour band and sensing knob. This use of this device allows patients to take a light shower.
Limitations
The limitation of this study was the small number of patients and might not represent the population at large. A much larger study would be needed to show the significance of this finding.
Declaration of interest
BenQ Materials Corporation sponsored the Anscare SIMO NPWT used for this study. The author has no conflicts of interest to declare.
References
1. de Alcântara Jones D, Filho WVN, de Souza Guimarães J et al (2016) The use of negative pressure wound therapy in the treatment of infected wounds. Case studies. Rev Bras Ortop 51(6): 646–51. https://dx.doi.org/10.1016%2Fj. rboe.2016.10.014
2. Apelqvist J, Willy C, Fagerdahl AM et al (2017) EWMA Document: Negative Pressure Wound Therapy – overview, challenges and perspectives. J Wound Care 26(3 Suppl 3):S1–113. https://doi.org/10.12968/ jowc.2017.26.sup3.s1
3. Birchenough SA, Gampper TJ, Morgan RF (2008) Special considerations in the management of pediatric upper extremity and hand burns. J Craniofac Surg 19(4):933–41. https://doi.org/10.1097/scs.0b013e318175f3f6
4. Borgquist O, Ingemansson R, Malmsjo M (2010) Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from −10 to −175 mmHg. Plast Reconstr Surg 125(2):502–9. https://doi.org/10.1097/prs.0b013e3181c82e1f
5. Bryan J. Moist wound healing: a concept that changed our practice. J Wound Care 2004;13(6):227–8. https://doi. org/10.12968/jowc.2004.13.6.26625
6. Cantero R, Rubio-Perez I, Leon M et al (2016) Negativepressure therapy to reduce the risk of wound infection following diverting loop ileostomy reversal: an initial study. Adv Skin Wound Care 29(3):114–8. https://doi. org/10.1097/01.asw.0000480458.60005.34
7. Chen SZ, Li J, Li XY, Xu LS (2005) Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg 28(3):211–7
8. Contractor D, Amling J, Brandoli C, Tosi LL (2008) Negative pressure wound therapy with reticulated open cell foam in children: an overview. J Orthop Trauma 2008;22(10
9. Fabian TS, Kaufman HJ, Lett ED et al (2000) The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg 66(12):1136-43 Suppl):S167–76
10. Gabriel A, Shores J, Heinrich C et al (2008) Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J 5(3):399–413. https://doi.org/10.1111/j.1742- 481X.2007.00423.x
11. Guo S, DiPietro, LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–29. https://doi. org/10.1177/0022034509359125
12. Huang C, Leavitt T, Bayer LR, Orgill DP (2014) Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 51(7):301–31. https://doi.org/10.1067/j. cpsurg.2014.04.001
13. Lavery LA, Murdoch DP, Kim PJ et al (2014) Negative Pressure Wound Therapy with low pressure and gauze dressings to treat diabetic foot wounds. J Diabetes Sci Technol 8(2):346–9. doi:10.1177/1932296813519012
14. Molnar JA, Simpson JL, Voignier DM et al (2006) Management of an acute thermal injury with subatmospheric pressure. J Burns Wounds 4:e5
15. Mooney JF 3rd, Argenta LC, Marks MW et al (2000) Treatment of soft tissue defects in pediatric patients using the V.A.C. system. Clin Orthop Relat Res 376:26–31
16. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38(6):553–62. https://doi. org/10.1097/00000637-199706000-00001
17. Morykwas MJ, David LR, Schneider AM et al (1999) Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil 20(1 Pt 1):15–21. https://doi. org/10.1097/00004630-199901001-00003
18. Mouës CM, Vos MC, van den Bemd GJ et al (2004) Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 12(1):11–17. https://doi.org/10.1111/j.1067- 1927.2004.12105.x
19. O’Leary DP, Peirce C, Anglim B et al (2017) Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: The P.I.C.O. Trial. Ann Surg 265(6):1082–6. https://doi.org/10.1097/ sla.0000000000002098
20. Passaretti D, Billmire DA (2003) Management of pediatric burns. J Craniofac Surg 14(5):713–8
21. Poehnert D, Hadeler N, Schrem H et al (2017) Decreased superficial surgical site infections, shortened hospital stay, and improved quality of life due to incisional negative pressure wound therapy after reversal of double loop ileostomy. Wound Repair Regen 25(6):994– 1001. https://doi.org/10.1111/wrr.12606
22. Schrank C, Mayr M, Overesch M et al (2004) [Results of vacuum therapy (V.A.C.) of superficial and deep dermal burns]. [Article in German] Zentralbl Chir 2004;129:59– 61. https://doi.org/10.1055/s-2004-822605
23. Scherer SS, Pietramaggiori G, Mathews JC et al (2008) The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 2008;122:786–97
24. Simman R, Forte R, Silverberg B, Moreira-Gonzales A (2004) A comparative histological pilot study of skin graft tack with tie-over bolster dressing versus vacuum assisted closure in a pig model. Wounds 16(2):76–80
25. Stamatas GN, Nikolovski J, Luedtke MA et al (2009) Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol 27(2):125–31. https://doi.org/10.1111 /j.1525-1470.2009.00973
26. Wackenfors A, Sjögren J, Gustafsson R et al (2004) Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 12:600–6. https://doi.org/10.1111/j.1067-1927.2004.12602.x
27. Wackenfors A, Gustafsson R, Sjögren J et al (2005) Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg 79(5):1724–1730. https://doi.org/10.1016/j. athoracsur.2004.10.053
28. Wayne PA, Krasner D, Rodenheaver D, Sibbald RG. Chronic wound care: a clinical source book for health care professionals. HMP Communications LLC,1996
This article is excerpted from the Wounds Asia 2021 | Vol 4 Issue 3 | by Wound World.


