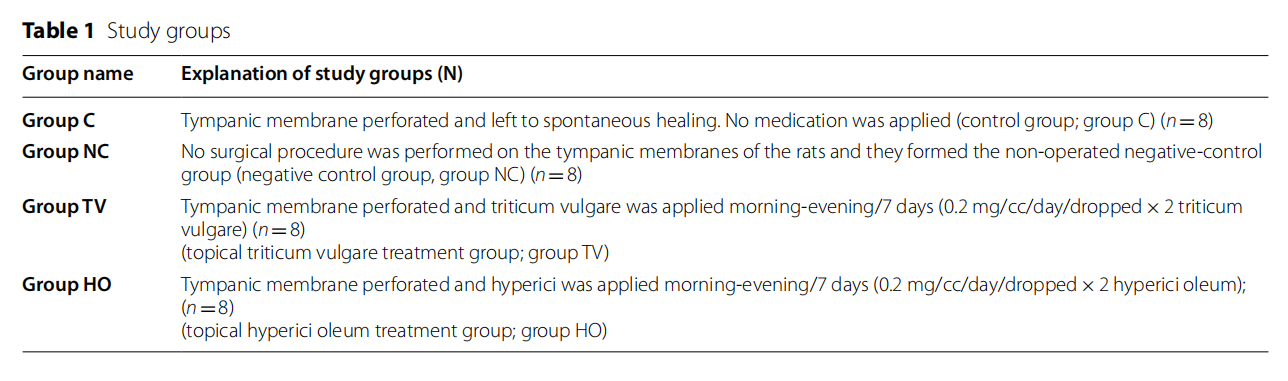
Background
Tympanic membrane (TM) perforation is a frequently seen clinical condition in ear-nose and throat practice. Although numerous factors play a role in its etiology, the most common causes are iatrogenic causes, trauma, or infections. Most traumatic TM perforations occur for reasons such as penetrating or blunt traumas, barotrauma, and excessive acoustic energy exchange [1]. TM perforations are typically classified as acute perforations, which tend to heal spontaneously according to the perforation time and as chronic perforations, which require to be treated with surgical methods. Traumatic TM perforations are considered acute perforations that may to heal spontaneously; however, chronic perforations may develop as a secondary progression to the trauma. For such perforations, spontaneous healing could not be observed and surgical intervention may be required [2].
As spontaneous healing occurs within 3–4 weeks in a great majority of the traumatic TM perforations, the clinical treatment approaches in practice are usually conservative. Thus, patients should be informed in detail about keeping their external auditory canal dry and clean, in order to prevent ear infections [3]. In the spontaneous healing period, closure signs in the perforation site should be closely monitored and materials such as rolling paper, grease, gelatin film, and silk may be used on the perforation site, under outpatient care conditions and if required for paper patch application, in order to accelerate the closure [3]. However, if spontaneous healing does not occur at the end of this period, various surgical procedures such as myringoplasty may be required to close the perforation. As in any other surgical process, such surgical interventions carry some risks, which has prompted researchers to investigate different options that may increase the effectiveness of the spontaneous healing process [1]. Tere are numerous studies in the literature that have investigated the positive or negative effects of various agents used in previous studies, on the healing of TM perforations. These agents include hyaluronic acid, epidermal growth factor, transforming growth factor, platelet-derived growth factor, platelet-rich plasma and Hypericum perforatum [4–6].
In a study conducted by Yaşar et al., [6] in 2016, the authors revealed the healing effect of Hypericum perforatum on TM perforation. Oleum hyperici (HO) is an extract prepared by means of olive oil from the flowers of Hypericum perforatum (references), and St. John’s wort oil, as it is known in popular medicine, is applied to heal wounds [7]. As Hyperici oleum (HO), Triticum vulgare (TV) is widely used in traditional medicine due to its tissue regeneration and accelerating properties. It has been proven in scientific studies that Hyperici oleum can be used safely as a topical agent [7]. Previous studies have shown that Triticum vulgare can be used safely as a topical agent [8]. TV takes place within numerous pharmacological agents that are used in wound healing. Various studies have revealed that TV significantly accelerates wound-healing processes and stimulates the cell chemotaxis and fibroblast activation [9].
When the studies on HO and TV in the literature are evaluated, the similar characteristics in both products can be highlighted. To the best of our knowledge, there is no clinical or experimental studies showing that TV is used in the treatment of TM perforations. Tus, the aim of the present study was to investigate whether or not the topical application of TV in experimentally induced traumatic TM perforations in rats has a positive effect on wound the healing process, as does HO.
Methods
Experimental animals
The randomized experimental procedure was used in this study in which the external auditory canals and tympanum of 30 female 16- to 18-week-old Wistar albino rats, having a mean weight of 230 ±10 g and which were anesthetized with 3 mg/kg Xylazin S.C. and 90 mg/kg Ketamine HCL S.C., were examined using an operation microscope (OPMİ Vario; Zeiss, Jena, Germany). After the rats with external and middle ear pathologies, such as infection in the external auditory canal, purulent discharge and pathology of the eardrum, were excluded from the study, a total of 24 rats were included in the study. Given that α =0.05, β=0.10, and (1-β)=0.90, it was decided to include 8 rats in each group and the power of the test was determined as p=0.9040.
Wistar albino rats (n =24) were randomly assigned to four experimental groups as group C (control group, n =8), group NC (negative control group, n=8), group TV ( Triticum vulgare, n=8), and group HO (Hyperici oleum, n =8) (Table 1). It was planned in the experimental protocol to exclude rats which died for any reason during the experimentation; however, no rat died during the study.
The rats were kept in steel cages under standard laboratory conditions (12-h light/dark cycle, 24±2 °C, 35–60% humidity) and fed with fresh feed without any nutrient limitation.
The trials were conducted in accordance with the National Institute of Health (NIH) Guide for the care and use of Laboratory Animals (NIH Publications No. 80–23 Revised 1996). The protocol of the study was approved by the Institutional Review and Animal Ethics Use Committee of XXX XXX University School of Medicine, and the study was conducted based on the accepted guidelines on the care and use of laboratory animals (Date: 03.09.2019; Decision number: 65202830–050.04.04–303).
Drug and chemicals
While Triticum vulgare was supplied from Orgamyra Cosmetic Care Products (Rosece wheat germ oil, rosece, no: 117, 30 ml; Orgamyra Cosmetic Care Products Industry Company, Ankara, Turkey), Hyperici Oleum was supplied from Zade Vital (Zade Vital, Zade Global Inc. Konya, Turkey; 100 ml).
Operation procedure and study protocol
The animals (n =24) were anesthetized with subcutaneous injections of ketamine hydrochloride (Ketalar ®, Pfizer Turkey, Istanbul, Turkey) of 90 mg/kg and xylazine hydrochloride (Rompun®, Bayer, Istanbul, Turkey) of 3 mg/kg. In the otomicroscopic examination, standard myringotomies with a 2-mm diameter were performed on the inferoposterior quadrant of the tympanic membrane, using a pick, approximately 10 min after the administration of anesthetic agent [10]. Metamizole of 20 mg/kg (Novalgine, 2 mL; Sanofi, Paris, France) was administered intramuscularly for pain. All surgical procedures were performed by the same surgeon (AB) ensuring the required sterilization conditions.
The same person performed all the drug administrations for the perforation in the right external auditory canal, twice a day for 7 days, within the same time period. Drugs were dropped with an injector on the external ear canal of the rats. The rats were randomized into four groups to investigate histopathological healing in the tympanic membrane. The procedure for each group is described below.
- In the first group, no surgical procedure was performed on the tympanic membranes of the rats and they formed the non-operated negative-control group (negative control group; group NC)
- In the second group, the infer oposterior quadrant of tympanic membranes of the rats was perforated and left to spontaneous healing. No medication was applied (control group; group C)
- In the third group, 1 cc of Triticum vulgare was dropped with an injector twice a day for 7 days on the external ear canal of the rats following myringotomies (topical Triticum vulgare treatment group; group TV)
- In the fourth group, 1 cc of Hyperici oleum was dropped with an injector twice a day for 7 days on the external ear canal of the rats following myringotomies (topical Hyperici oleum treatment group; group HO
-
Seven days after the completion of the medical treatments, all the rats were sacrificed with intraperitoneal injections of Pentothal sodium (200 mg/kg). After decapitation, the tympanic bullae of the rats were removed. Tissue samples were placed in containers without group names. Each of the biopsy materials were collected and submitted for histopathological examination.
Histopathological examinations
The histological analyses were performed by one histopathologist (ZDŞİ) who was blind to the samples. Tissue samples taken were fixed for 24–48 h by coding in 10% buffered neutral formalin, in containers with out the group names. The tissue was then softened by keeping it in a decalcification solution for 30 days in a controlled manner. From the tympanic membrane, cartilage excision of both external auditory canals was performed with a 5×5-mm through-cut excision including the experimental perforation border. Softened tissues were passed through a tissue follow-up and paraffin was blocked. From the paraffin blocks, 3–5-µm sections were taken and evaluated by using a light microscope (Olympus BX51, Tokyo, Japan) after staining with hematoxylin eosin (H&E).
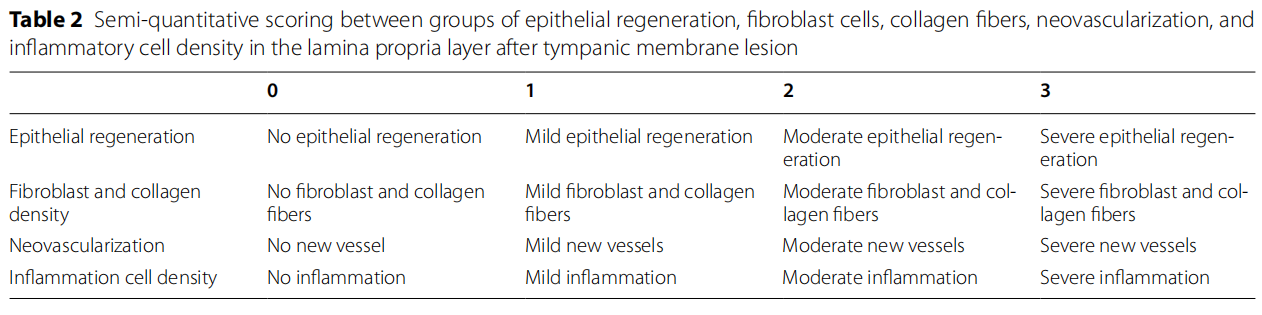
Based on the studies of Erkilet et al., [5] and Bilge et al., [8], regeneration of the perforation epithelium, the presence of fibroblast and collagen, the density of capillary veins, and the presence of infammatory cells were histopathologically compared among the groups and scored between 0 and 3 (0: no, 1: mild, 2: moderate, 3: severe) (Table 2). The results obtained were evaluated statistically.
Statistical analysis
The use of experimental animals has been restricted in Turkey, following a decision published in the Official Gazette of the Republic of Turkey on February 15, 2014. In accordance with this and a decision from the Ethics Committee of Sivas Cumhuriyet University, the number of rats to be used in studies conducted within our university with experimental animals has been determined as eight per experimental group. The data was analyzed by using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL) for Windows, version 22.0. Since the parametric test assumptions could not be performed to evaluate the data (n<30), the Kruskal–Wallis test was used to compare data obtained from more than two independent groups and the Mann–Whitney U test was used to find the groups making difference when a significance decision was given in the analysis result. The level of significance was set at the value of 0.05.
Results
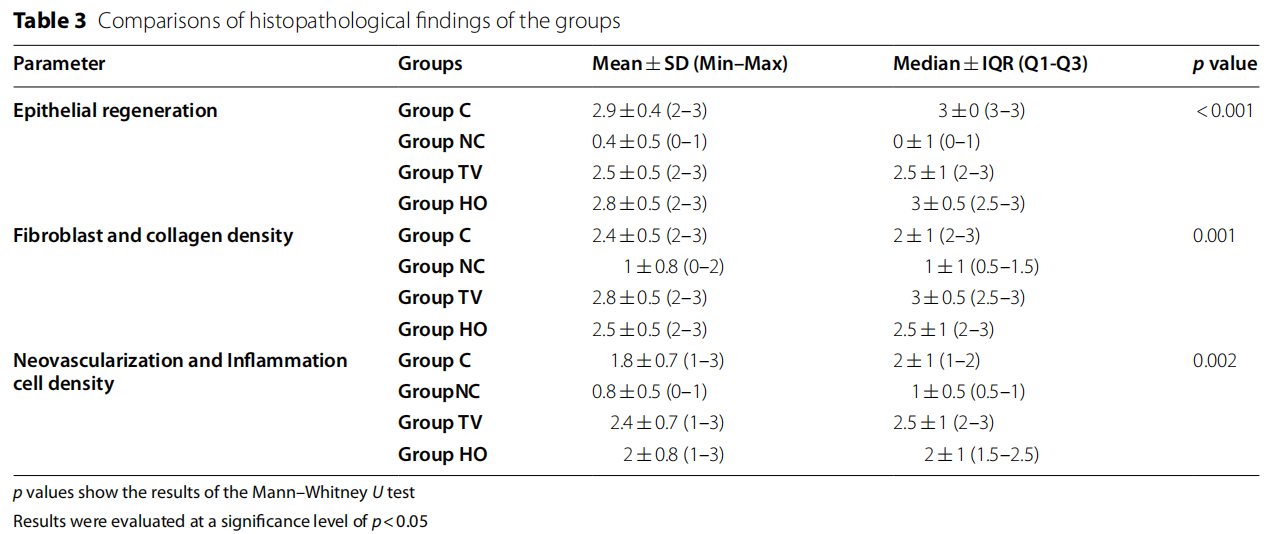
When the groups were evaluated in terms of epithelial regeneration in the tissue samples obtained in the study, no statistically significant difference was found between groups C, TV, and HO (p>0.05). In terms of the epithelial regeneration mean scores, the difference between groups C, TV, and HO and group NC was statistically significant and the mean values obtained in group NC were lower than the other three groups (p<0.05). When the groups were examined in terms of density of fibroblast and collagen, no statistically significant difference was found between groups C, TV, and HO (p>0.05).
The mean values found in group NC were lower than all the three groups, and the difference was statistically significant (p<0.05). No significant difference was found between groups C, TV, and HO, when a statistical evaluation was conducted in terms of neovascularization and inflammation cell density scores (p>0.05). In terms of the neovascularization and inflammation cell density mean scores, the difference between groups C, TV, HO and group NC was statistically significant and the mean values obtained in group NC were lower than the other three groups (p<0.05).
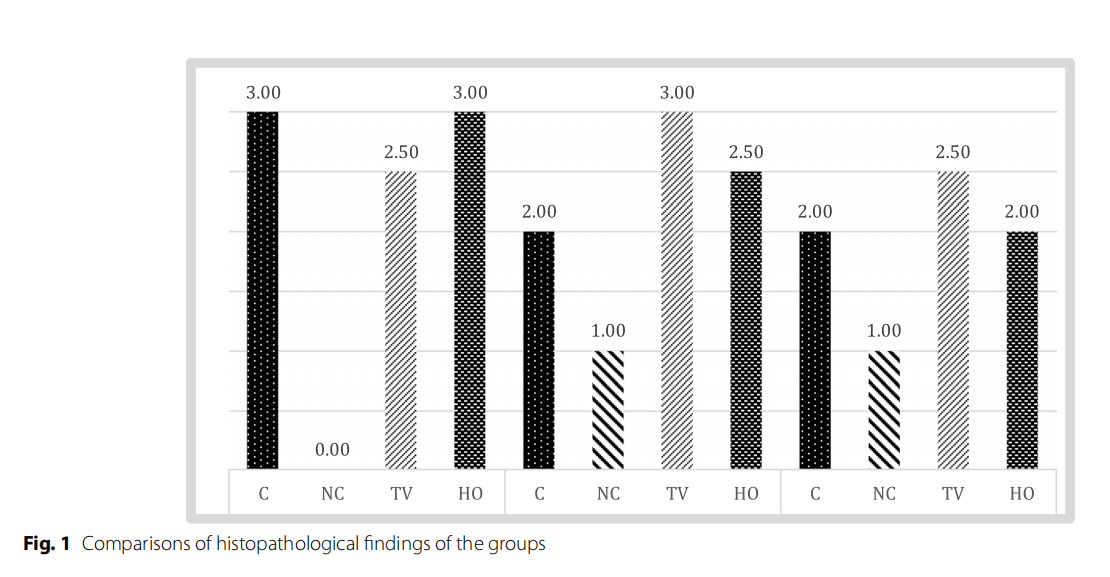
Table 3 and Fig 1 show the histopathological findings of each group.
Light microscopic evaluation
In the histological evaluation, reepithelization was better in the group HO than the group TV on the 7th day after perforation; however, it was observed that there was no significant difference in the group HO when compared to
group C, and both experimental groups had better reepithelization capacity than group NC.
In the evaluation performed in terms of the subepithelial fibroblast cells, density of collagen fibers, formation of new veins, and inflammatory cell accumulation, it was observed that group TV was better than group HO, and in particular, group TV had a significant difference from group C histopathologically. Also, while the perforation site was completely closed on the 7th day in groups HO and TV, it was closed with a thinner structure in the group that was left to heal spontaneously (Fig. 2).
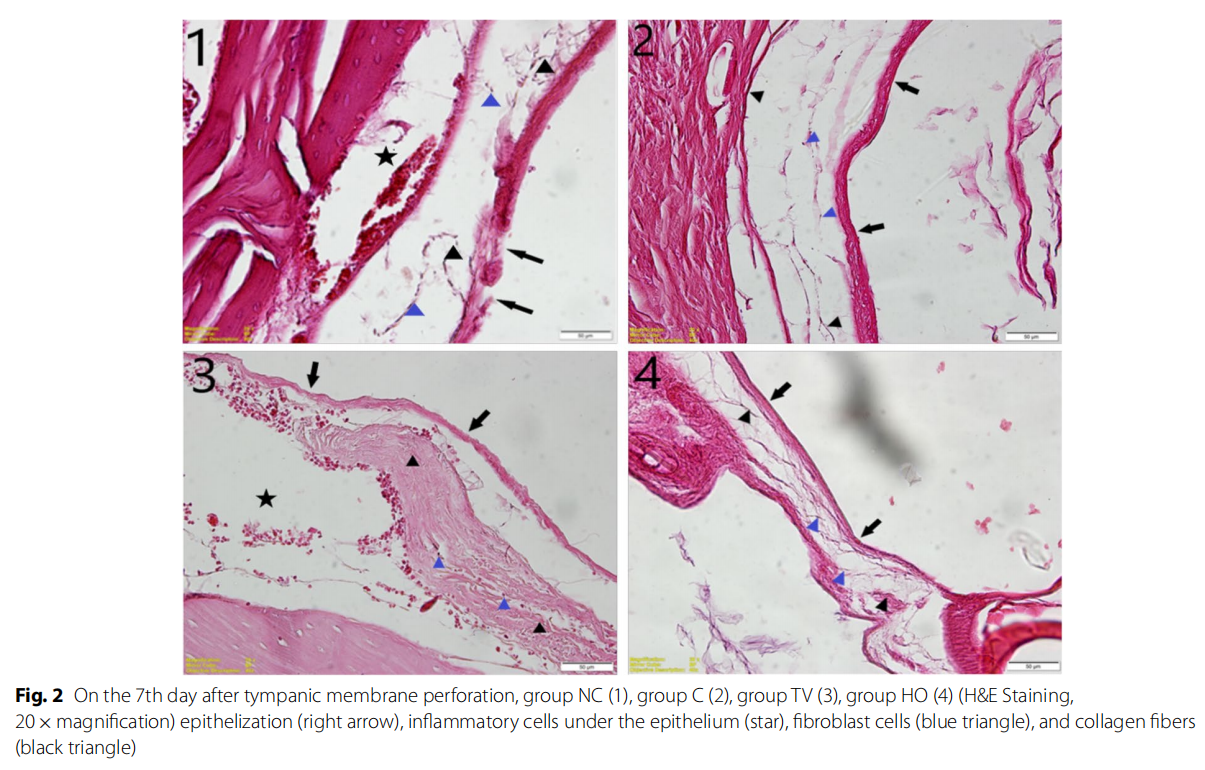
Discussion
From outwards to inwards, TM histopathologically consists of three different layers as ectoderm-originated keratinized squamous epithelium, mesodermally originated lamina propria, and endodermally originated mucosal layer [11]. TM perforation, which occurs due to numerous pathological reasons, can heal spontaneously. Tis occurs depending on the reproduction and migration of the squamous epithelium on the outer layer of TM. Tere are some differences between wound healing in other tissues and TM wound healing. For example, hemostasis and inflammation stages show similarity, whereas proliferation and migration stages are completely different. While the wound is primarily closed by the granulation tissue in other tissues, closure in TM starts with the squamous epithelium layer [12]. Histopathological changes occurring in TM after perforation are detailed in the studies [13]. Inflammatory response is the initial indicator after perforation: inflammatory cells quickly accumulate at the sides and under the perforation site, followed by epithelial proliferation. When TM is perforated, epithelial cells show a rapid mitotic activity for reepithelization and they try to close the perforation site. As the epithelium takes form to close the proliferation site, it is thin at the beginning and gradually becomes thicker. Afterwards, the migration of keratocyte progenitor cells is initiated to the perforation site via the veins that are newly formed. These cells develop later at the edge of perforation, and epithelial bridges start to form from this layer towards the center of perforation. While the new veins are formed on the one hand, inflammation cells pull the fibroblasts to this site quickly on the other hand, and fibrous layer is formed via the fibroblast activation. Fibroblasts then form collagen fibers and provide the connective tissue support. A perforation site that has been reduced with the help of the inflammatory cells that have accumulated at the beginning of the wound healing process, closes in approximately 7–10 days [10, 12]. While many tissues in the body have a matrix that supports epithelial regeneration and provides access to nutrients with cells that play a role in tissue repair, this matrix structure does not exist under TM. A continuous migration of the epithelial layer is present towards the superior side of TM and this reveals that TM can repair itself. Despite this perfect repair mechanism, one of the emerging and frequently encountered condition that is secondary to infections or traumas in ear-nose-throat practice is, unfortunately, TM perforation. Traumatic TM perforations are a common clinical condition encountered in otology practice. Due to the high rates of spontaneous healing, to this day, no consensus has been reached regarding treatment approaches. While some authors recommend waiting for spontaneous healing, some others recommend one of medical and/or surgical treatments immediately. In large perforations, spontaneous closure periods are lengthier when compared to small TM perforations. In addition, spontaneous closure may not occur in numerous cases. Thus, some researchers have highlighted that various biological materials could be beneficial in large perforations, in order to accelerate wound healing and provide spontaneous healing [14–17]. However, it is currently still unclear which treatment is more appropriate in the cases of traumatic TM perforation and studies on this matter are ongoing.
TV and HO are frequently used in traditional medicine due to their tissue repair-accelerating effect [18,19]. TV and HO induce the active cells in wound healing process, especially fibroblast and endothelium, and thus, affect wound healing [18, 20]. Tus, the fact that TM perforations can also have positive effects on the healing process should not be neglected. Tere are a limited number of studies for HO regarding their use as the healing factor in TM perforation, whereas no study on TV has been found.
In the study conducted by Yaşar et al. using Hypericum perforatum L. as a curative agent in TM perforations, a spontaneous healing group and a medical treatment group were compared in terms of histopathological findings: they found that Hypericum perforatum L. was effective in would healing [6]. In their study, Eğilmez et al. showed that oral or topical application of Hypericum perforatum L. prevented myringosclerosis and significantly decreased inflammation and fibrosis in the lamina propria of tympanic membranes. Another result of the present study is that Hypericum perforatum L. reduced the thickness of TM [21].
In conclusion, in our study, we evaluated the effects of two compounds whose efficacy in TM perforations is little known and/or unknown and found a widespread use in traditional medical practices due to their positive effects on wound healing. Our findings indicated that both HO and TV applications in perforation sites had better results than spontaneous healing in terms of reepithelization. Another important finding was that based on the results of histopathological assessment. The increase in epithelization observed in the perforation site in the rats to which TV and HO were administered, and healing in TM perforation had nearly reached the same epithelial thickness with the rats in the control group with intact tympanic membrane integrity. Tis suggested that TV and HO accelerated both wound healing in the perforation site and provided closure with a tissue that is histopathologically quite similar to the normal tissue.
It is known that high inflammation at the beginning of wound healing is an indicator of a strong response to healing. Also, a fibrotic connective tissue under the epithelium is formed via the presence of inflammatory cells in the wound healing process in TM. This fibrotic component is different from the granulation tissue that is observed in normal wound healing. Tus, the organization of the collagen fibers under the epithelium and fibroblast density play a role in the structuring of TM perforation area [10, 22]. In the present study, it was observed that inflammatory cell response significantly increased in both TV and HO groups, compared to spontaneous healing group, although this increase was observed mostly in the TV group. In addition, the presence of regular collagen fibers and dense fibroblasts was observed in the TV and HO groups. These findings showed that both practices had positive effects on wound healing.
When the findings of the present study were evaluated as a whole, topical HO and TV practices had positive effects on healing of traumatic TM perforation in rats. Furthermore, when the contributions of TV and HO on wound healing were assessed in terms of reepithelization, inflammatory cell localization, formation of new veins, organization of collagen fibers, and fibroblast cell density, it was seen that TV was more effective than HO. As a result, if one of both practices should be preferred in TM perforations, TV should be selected.
Our study had some limitation, one of which is that only the effects of TV and HO on acute perforations were evaluated. Also, the perforation size formed during the trial was small, and when the perforation size is increased in further studies, we consider that it will be better suitable to assess its effects on healing. Besides the perforation size, it should also be noted that the localization of the perforation site also had a significant effect on healing. In the study, perforation was conducted only in one site in all the rats, in an effort to provide standardization. However, it is suggested that further studies on the effectiveness of TV and HO in perforations at different localizations will be beneficial. Another significant limitation is that the effects of TV on hearing were not assessed. More comprehensive studies making electrophysiological evaluations in terms of ototoxicity are needed in the future.
Conclusion
This is the first study investigating the potential curative role of TV in an experimental rat model of tympanic membrane perforation. Considering the findings, it was concluded that TV can be more effective than HO on wound healing in TM perforation. In addition, it is considered that it may be an alternative treatment option among the clinical practices in otology, after comprehensive studies on ototoxicity have been conducted, due to its cost-effective, effective, and straightforward application in the treatment of TM perforations.
Acknowledgements
Not applicable
Authors’ contributions
Concept—E.E.A, A.B., Z.D.Ş.İ, and K.D.; Design—E.E.A, K.D., A.B., and Z.D.Ş.İ.; Supervision—E.E.A, A.B., and Z.D.Ş.İ.; Resource—E.E.A; Materials—A.B., Z.D.Ş.İ, and B.B.D.; Data collection and/or processing—A.B., K.D., Z.D.Ş.İ, and B.B.D.; Analysis and/or interpretation—E.E.A, Z.D.Ş.İ, and A.B.; Literature search—K.D., A.B., Z.D.Ş.İ., and B.B.D.; Writing—E.E.A, Z.D.Ş.İ, and A.B.; Critical reviews—E.E.A and Z.D.Ş.İ. The authors read and approved the final manuscript.
Funding
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The protocol of the study was approved by the Institutional Review and Animal Ethics Use Committee of Sivas Cumhuriyet University School of Medicine and the study was carried out based on the accepted guidelines on the care and use of laboratory animals (Date: 03.09.2019; Decision number: 65202830–050.04.04–303).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Received: 30 November 2022 Accepted: 12 January 2023
Published online :27 January 2023.
References
1. Huang J, Teh BM, Eikelboom RH, Han L, Xu G, Yao X, Hu Y, Zheng M, Shen Y (2020) The effectiveness of bFGF in the treatment of tympanic membrane perforations: a systematic review and meta-analysis. Otol Neurotol 41(6):782–790. https://doi.org/10.1097/MAO.0000000000 002628
2. Huang J, Shi Y, Wu L, Lv C, Hu Y, Shen Y (2021) Comparative effcacy of platelet-rich plasma applied in myringoplasty: a systematic review and meta-analysis. PLoS One 16(1): e0245968: https://doi.org/10.1371/journpone. 0245968.
3. Lee JH, Lee JS, Kim DK, Park CH, Lee HR (2015) Clinical outcomes of silk patch in acute tympanic membrane perforation. Clin Exp Otorhinolaryngol 8:117–122. https://doi.org/10.3342/ceo.2015.8.2.117
4. Kaftan H, Herzog M, Miehe B, Hosemann W (2006) Topical application of transforming growth factor-β1 in acute traumatic tympanic membrane perforations: an experimental study in rats. Wound Repair Regen 14:453–466
5. Erkilet E, Koyuncu M, Atmaca S, Yarim M (2009) Platelet-rich plasma improves healing of tympanic membrane perforations: experimental study. J Laryngol Otol 123(5):482–487. https://doi.org/10.1017/S0022 215108003848. (Epub 2008 Oct 28)
6. Yaşar M, Kaya A, Karaman H, Kavugudurmaz M, Polat H, Sağıt M, Özcan İ (2016) Potential curative role of Hypericum perforatum in an experimental rat model of tympanic membrane perforation. J Int Adv Otol 12(3):252–256. https://doi.org/10.5152/iao.2016.1612
7. Lyles James T., Kim A, Nelson K, Bullard-Roberts Angelle L., Hajdari A, Mustafa B, Quave Cassandra L (2017) The chemical and antibacterial evaluation of St. John’s wort oil macerates used in Kosovar traditional medicine. Front Microbiol 8: 1639: https://doi.org/10.3389/fmicb.2017. 016391
8. Final Report of the Safety Assessment for Wheat Flour and Wheat Starch (1980) Journal-of-Environmental-Pathology-and-Toxicology. USA 4(4):19–32
9. Sanguigno L, Casamassa A, Funel N, Minale M, Riccio R, Riccio S, Boscia F,Brancaccio P, Pollina LE, Anzilotti S,Renzo GD, Cuomo O (2018) Triticum vulgare extract exerts an anti-inflammatory action in two in vitro models of inflammation in microglial cells. PLoS One 13(6): e0197493:https://doi. org/10.1371/journ al.pone.0197493.
10. Bilge A, Gunes A, Dagli M, Koybasioglu FF, Guvey A (2016) (2016) The impact of topical and systemic enoxaparin sodium use on traumatic tympanic membrane perforation and myringosclerosis. Eur Arch Otorhinolaryngol 273(10):3035–3041. https://doi.org/10.1007/ s00405-016-3901-0
11. Yamashita T (1985) Histology of the tympanic perforation and the replacement membrane. Acta Otolaryngol 100:66–71
12. Makuszewska M, Sokolowska M (2015) Enhanced expression of hepatocyte growth factor in the healing of experimental acute tympanic membrane perforation. Int J Pediatr Otorhinolaryngol 79:987–992
13. Araujo MM, Musrashima AAB (2015) The topical use of insulin accelerates the healing of traumatic tympanic membrane perforations. Laryngoscope 126:156–162
14. Li X, Zhang H, Zhang Y (2020) Repair of large traumatic tympanic membrane perforation using ofloxacin otic solution and gelatin sponge. Braz J Otorhinolaryngol 88(1):9–14
15. Gür ÖE, Ensari N, Öztürk MT, Boztepe OF, Gün T, Selçuk ÖT, Renda L (2016) Use of a platelet-rich fibrin membrane to repair traumatictympanic membrane perforations: a comparative study. Acta Otolaryngol 136:1017–1023
16. Sayin I, Kaya KH, Ekizoğlu O, et al (2013) A prospective controlled trial comparing spontaneous closure and Epifilm® patching in traumatic tympanic membrane perforations. Eur Arch Otorhinolaryngol 270:2857–2863. https://doi.org/10.1007/s00405-012-2331-x
17. Silveira FC, Pinto FC, Caldas Neto Sda S, Leal Mde C, Cesário J, Aguiar JL (2016) Treatment of tympanic membrane perforation usingbacterial cellulose: a randomized controlled trial. Braz J Otorhi-nolaryngol 82:203–208
18. Tito A, Minale M, Riccio S, Grieco F, Colucci MG, Apone F (2020) A Triticum vulgare extract exhibits regenerating activity during the wound healing process. Clin Cosmet Investig Dermatol 13:21–30. https://doi.org/10. 2147/CCID.S216391
19. Quave CL (2018) Wound healing with botanicals: a review and future perspectives. Curr Dermatol Rep 7(4): 287–295: https://doi.org/10. 1007/s13671-018-0247-4. Epub 2018 Oct 25. PMID: 31106027; PMCID:
20. Bridi H, Beckenkamp A, Maurmann N, Elingson B, Bufon A, Pranke P, von Poser GL (2021) Phloroglucinol derivatives from Hypericum species induce in vitro proliferation of cells involved in the wound healing process. Nat Prod Res 35(22):4648–4652
21. Eğilmez OK, Kökten N, Ekici AI, Kalcıoğlu MT, Yesilada E, Tekin M (2015) The effect of Hypericum perforatum L. (St. John’s Wort) on prevention of myringosclerosis after myringotomy in a rat model. Int J Pediatr Otorhinolaryngol. 79(7): 1128–1134: https://doi.org/10.1016/j.ijporl.2015.05.009. Epub 2015 May 17. PMID: 26022750.
22. Lou ZC, Lou ZH (2017) A moist edge environment aids the regeneration of traumatic tympanic membrane perforations. J Laryngol Otol 131(7):564–571. https://doi.org/10.1017/S0022215117001001
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is excerpted from the The Egyptian Journal of Otolaryngology (2023) 39:26 by Wound World.


