文献精选


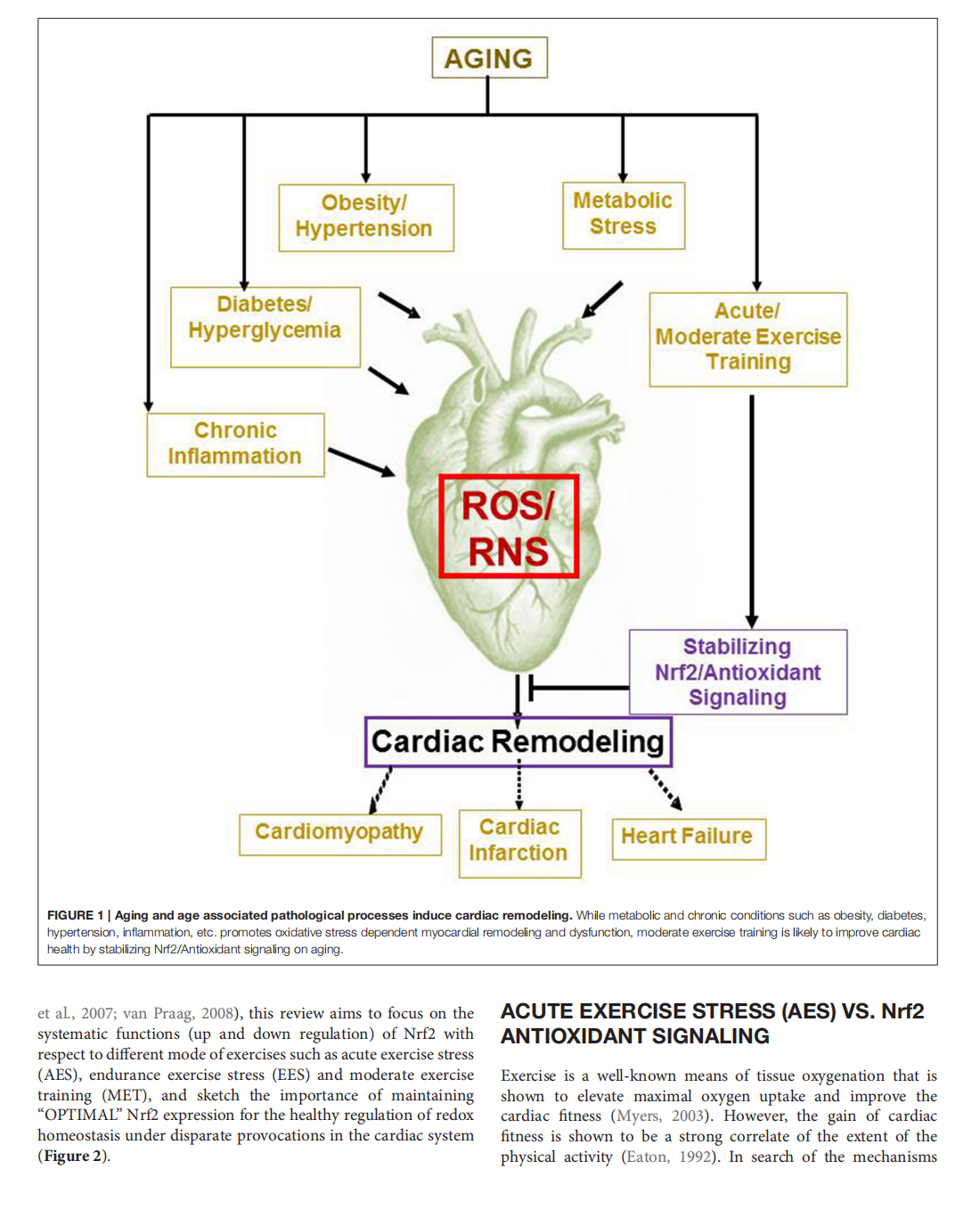




This article is excerpted from the 《Frontiers in Physiology》 by Wound World










This article is excerpted from the《Frontiers in Surgery》by Wound World

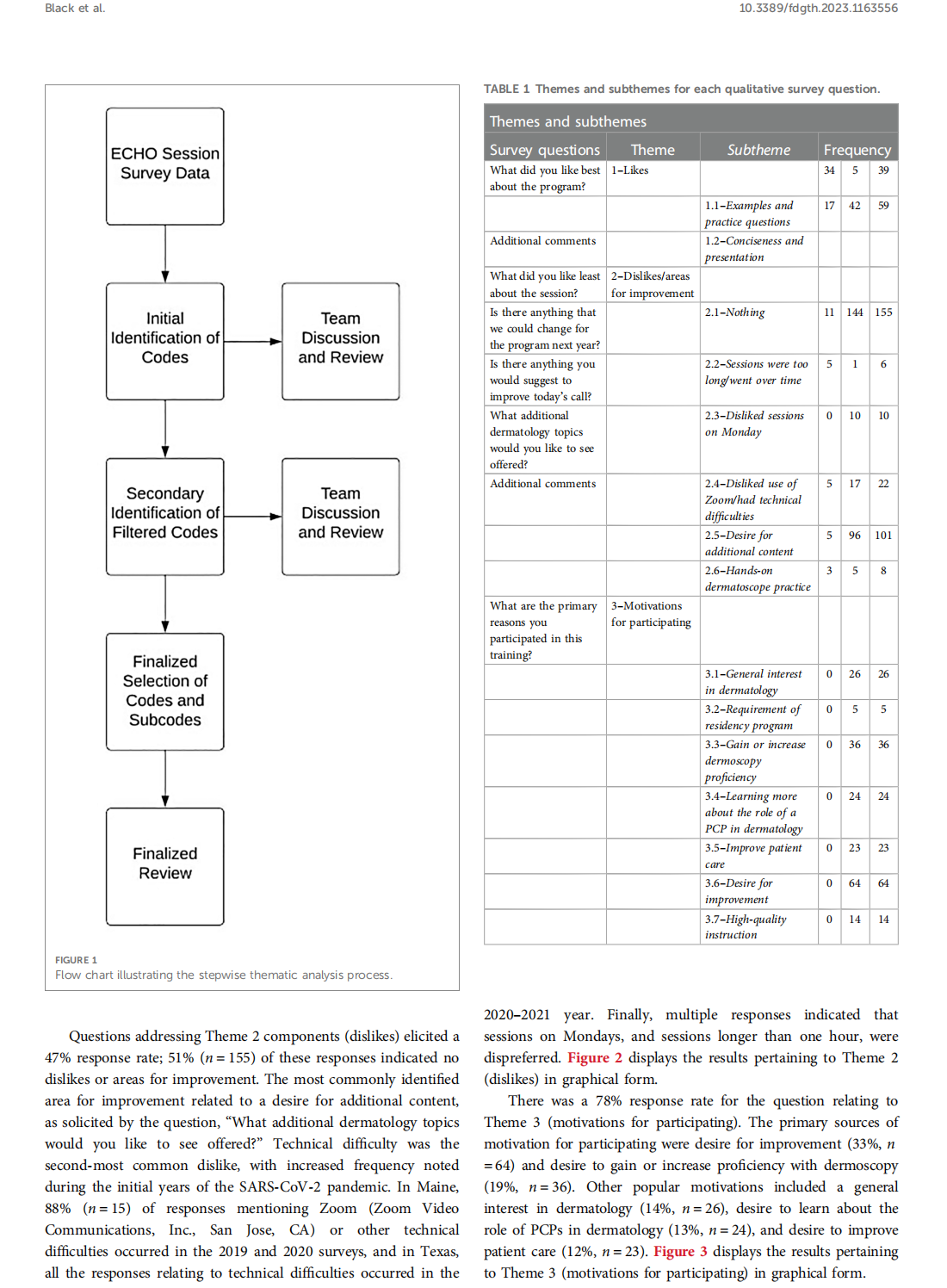

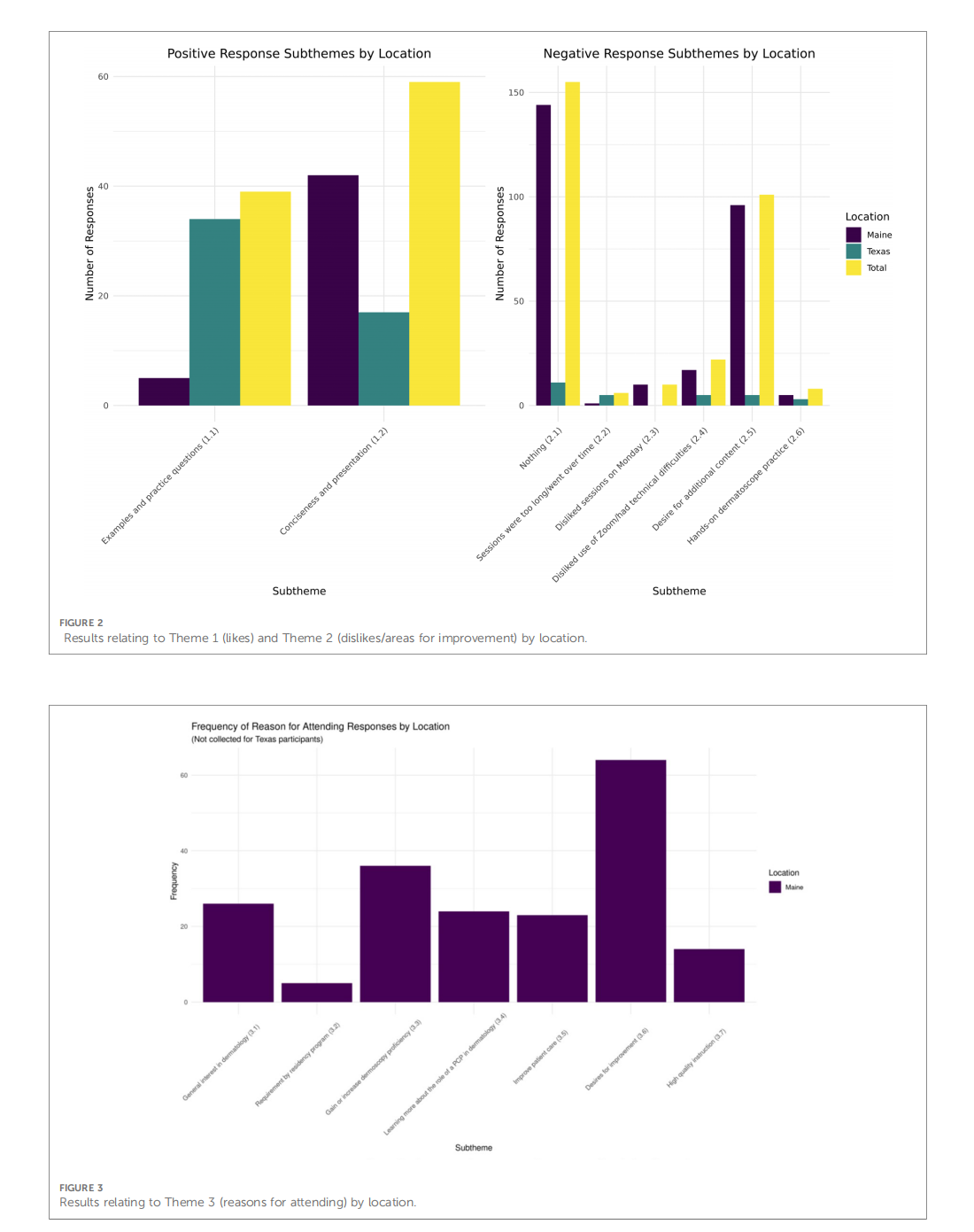


This article is excerpted from the 《Frontiers in Digital Health》 by Wound World






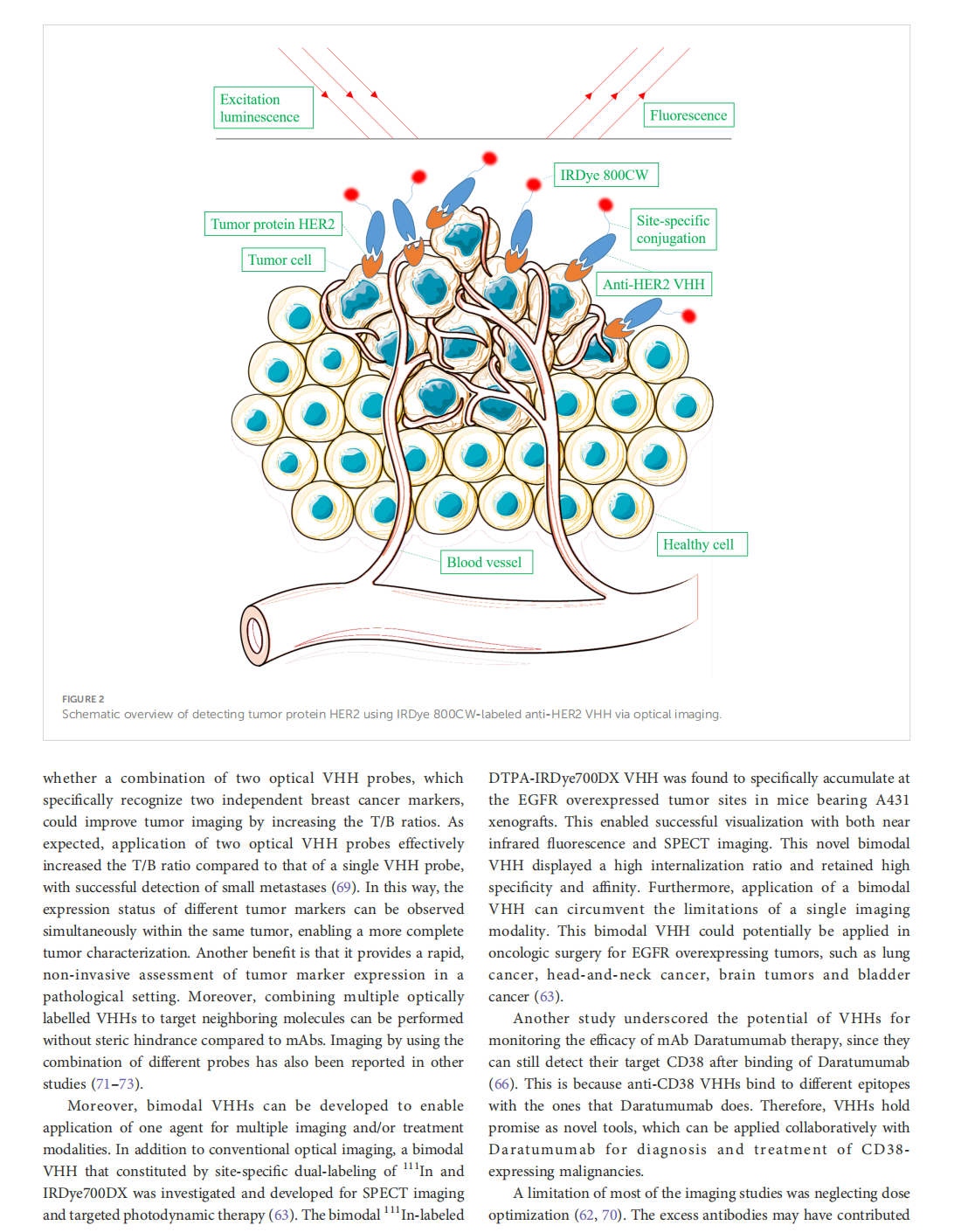











This article is excerpted from the 《Frontiers in Oncology》 by Wound World




